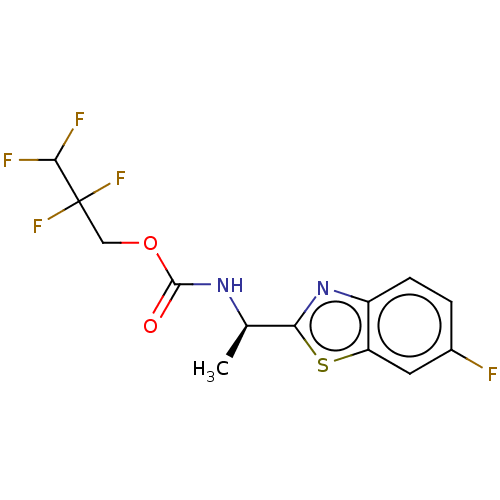
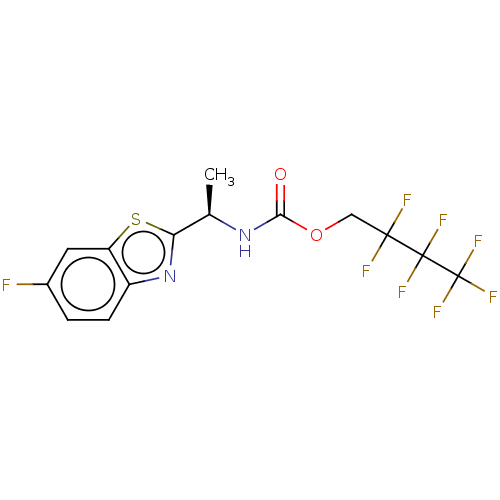
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
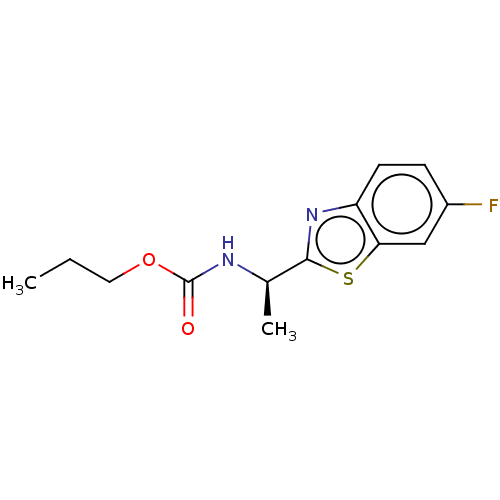
Affinity DataIC50: 6.06E+3nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
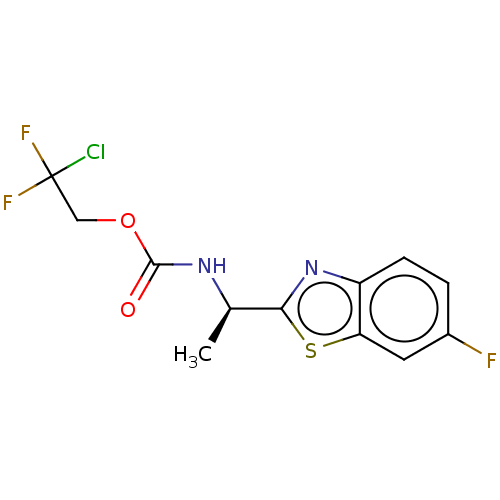
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
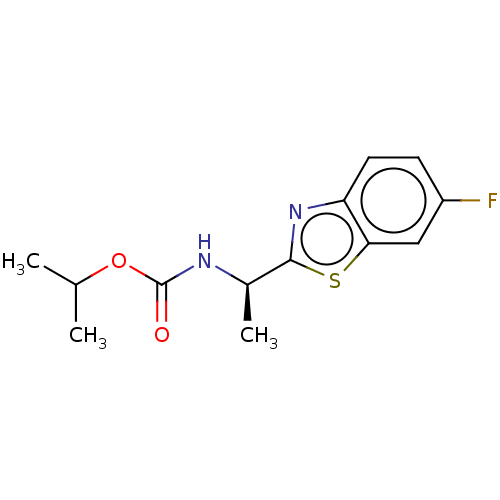
Affinity DataIC50: 6.75E+3nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
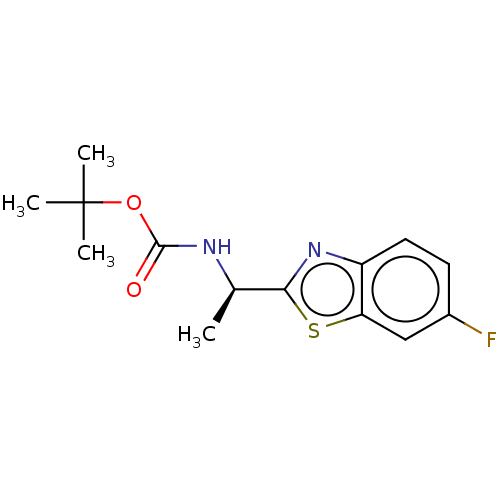
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
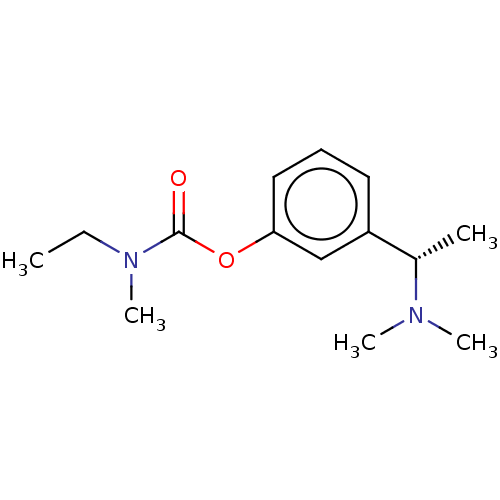
Affinity DataIC50: 8.14E+3nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 8.31E+3nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
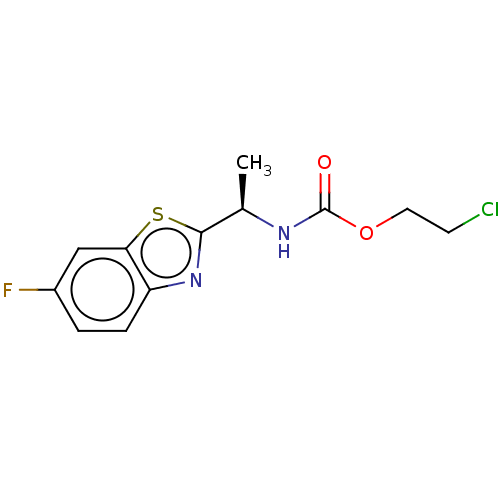
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
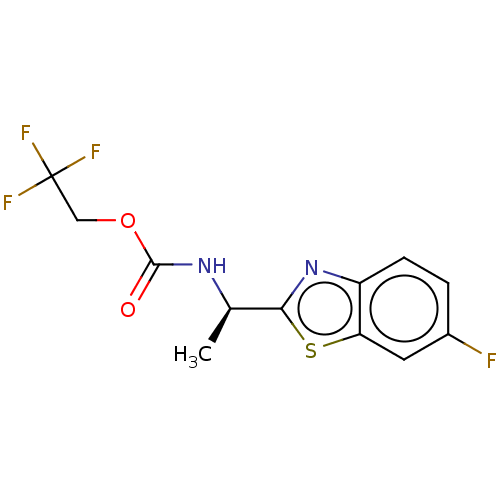
Affinity DataIC50: 9.26E+3nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.05E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.14E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.27E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.29E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.31E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.36E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 1.65E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.01E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.31E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.56E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.46E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.84E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.84E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.88E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.02E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 4.05E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.09E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.12E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 5.61E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 5.62E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.34E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 6.63E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.45E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 8.05E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 8.19E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 9.03E+4nMAssay Description:Inhibition of equine serum BChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Electrophorus electricus (Electric eel))
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataIC50: 9.22E+4nMAssay Description:Inhibition of electric eel AChE by spectrophotometric-based Ellman's methodMore data for this Ligand-Target Pair
TargetAcetylcholinesterase(Tetronarce californica (Pacific electric ray) (Tor...)
University Of Pardubice
Curated by ChEMBL
University Of Pardubice
Curated by ChEMBL
Affinity DataKoff: 0.0333s-1Assay Description:Pseudo-irreversible inhibition of Torpedo californica AChE using acetylthiocholine as substrate by cornish bowden plot analysisMore data for this Ligand-Target Pair