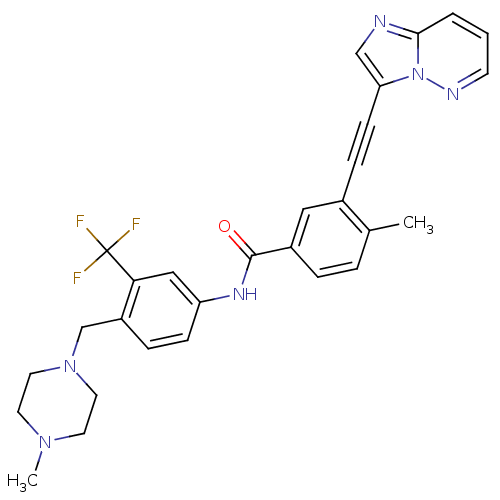
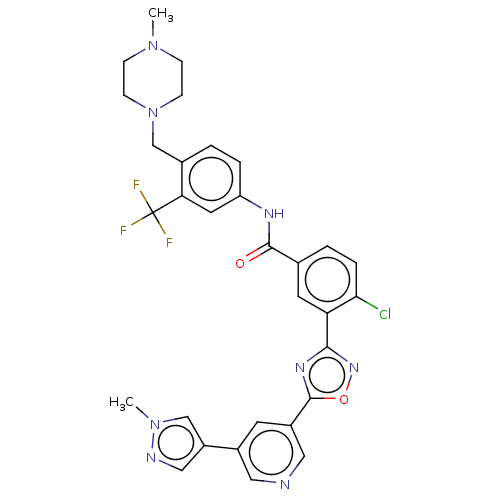
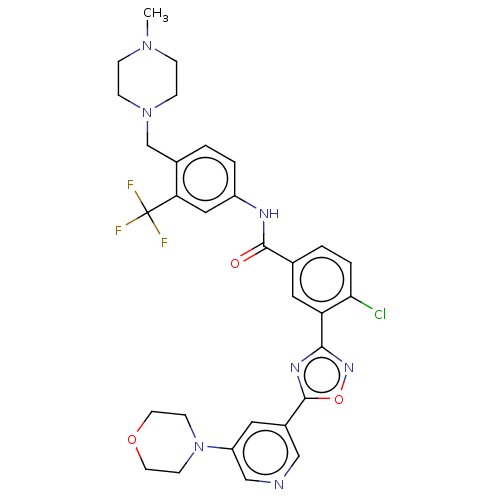
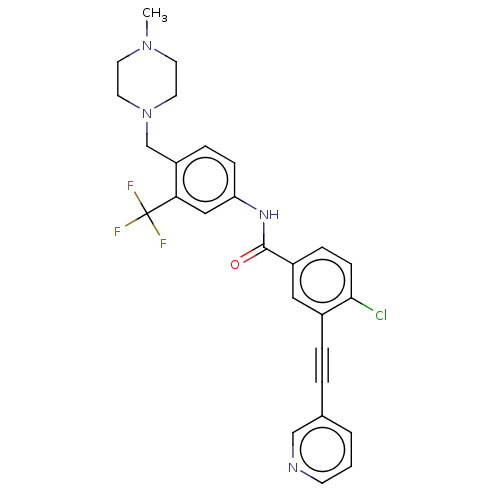
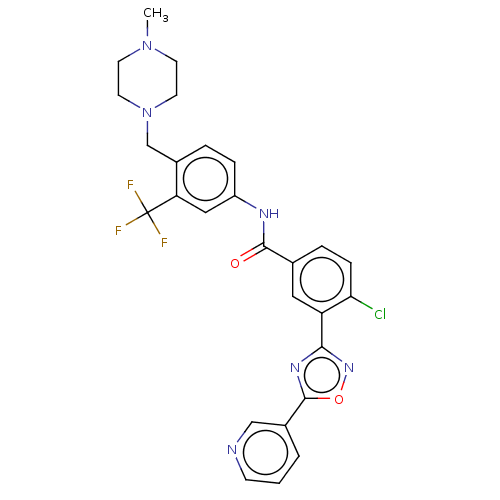
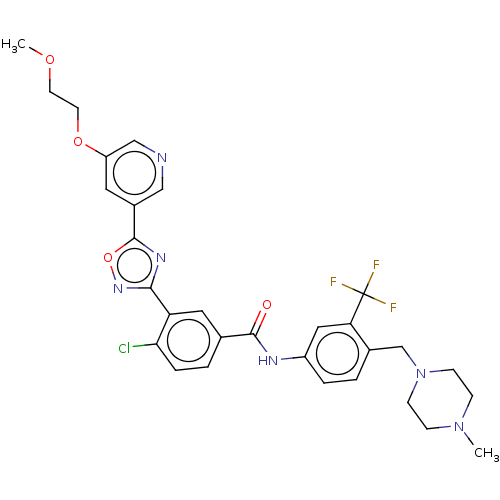
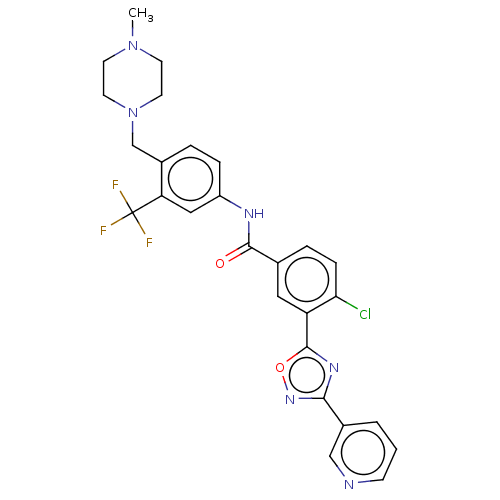
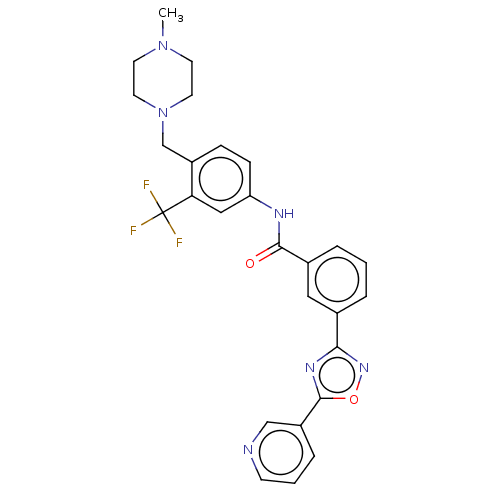
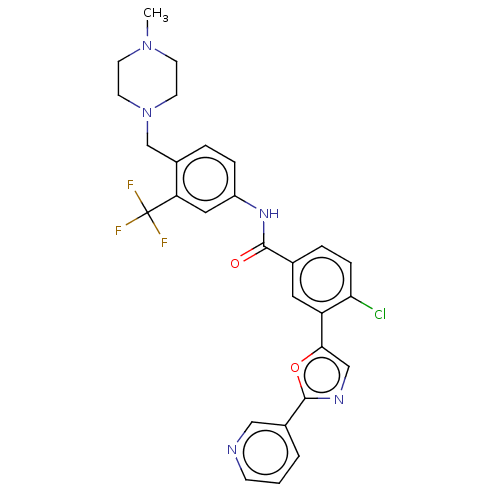
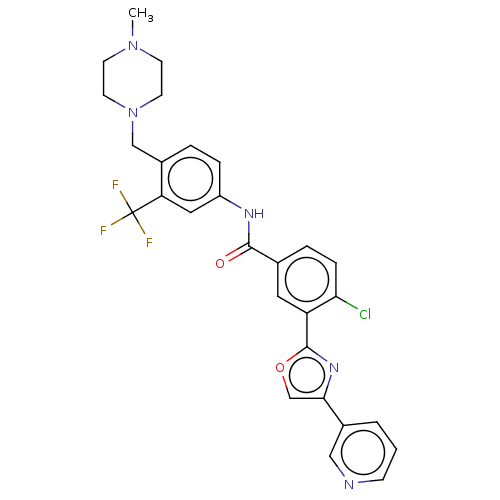
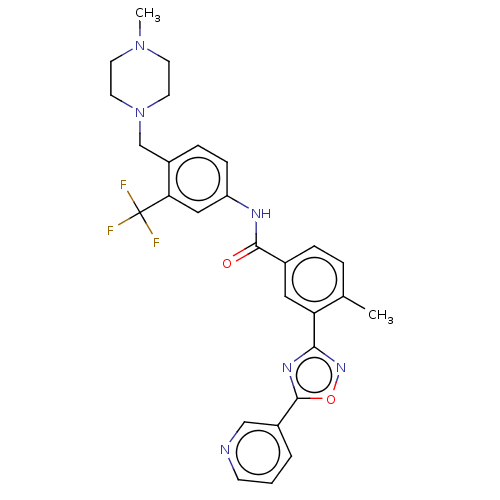
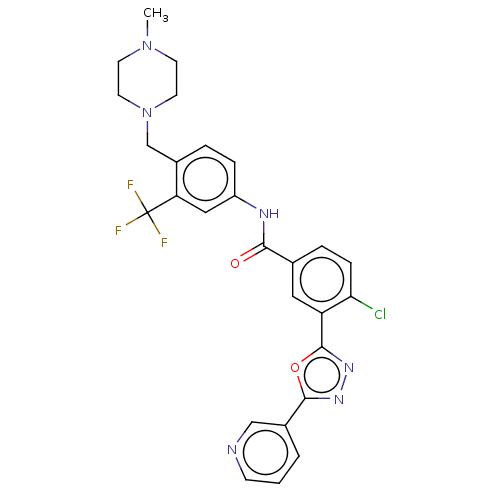
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 0.900nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 1.80nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 2.10nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 2.5nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 7.30nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 11nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 12nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 18nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 19nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 21nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 27nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 108nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 138nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetCoiled-coil domain-containing protein 6/Proto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 180nMAssay Description:Inhibition of CCDC6/RET (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation after 72 hrs by MTT assayMore data for this Ligand-Target Pair
TargetProto-oncogene tyrosine-protein kinase receptor Ret(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 342nMAssay Description:Inhibition of RET (unknown origin) using poly[Glu:Tyr] (4:1) as substrate after 60 mins by ELISAMore data for this Ligand-Target Pair
TargetCoiled-coil domain-containing protein 6(Homo sapiens (Human))
Ocean University Of China
Curated by ChEMBL
Ocean University Of China
Curated by ChEMBL
Affinity DataIC50: 407nMAssay Description:Inhibition of CCDC6/RET (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation after 72 hrs by MTT assayMore data for this Ligand-Target Pair