Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Aromatase
Ligand
BDBM8871
Substrate
BDBM8885
Meas. Tech.
CYP11B Assay
IC50
190±n/a nM
Citation
 Ulmschneider, S; Muller-Vieira, U; Mitrenga, M; Hartmann, RW; Oberwinkler-Marchais, S; Klein, CD; Bureik, M; Bernhardt, R; Antes, I; Lengauer, T Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: potent inhibitors of aldosterone synthase. J Med Chem 48:1796-805 (2005) [PubMed] Article
Ulmschneider, S; Muller-Vieira, U; Mitrenga, M; Hartmann, RW; Oberwinkler-Marchais, S; Klein, CD; Bureik, M; Bernhardt, R; Antes, I; Lengauer, T Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: potent inhibitors of aldosterone synthase. J Med Chem 48:1796-805 (2005) [PubMed] Article More Info.:
Target
Name:
Aromatase
Synonyms:
ARO1 | Aromatase | CP19A_HUMAN | CYAR | CYP19 | CYP19A1 | CYPXIX | Cytochrome P-450AROM | Cytochrome P450 19A1 | Cytochrome P450 2C19 | Cytochrome P450-C19 (CYP19) | Estrogen synthetase | FL cytokine receptor precursor | P-450AROM
Type:
Enzyme
Mol. Mass.:
57888.92
Organism:
Homo sapiens (Human)
Description:
P11511
Residue:
503
Sequence:
MVLEMLNPIHYNITSIVPEAMPAATMPVLLLTGLFLLVWNYEGTSSIPGPGYCMGIGPLISHGRFLWMGIGSACNYYNRVYGEFMRVWISGEETLIISKSSSMFHIMKHNHYSSRFGSKLGLQCIGMHEKGIIFNNNPELWKTTRPFFMKALSGPGLVRMVTVCAESLKTHLDRLEEVTNESGYVDVLTLLRRVMLDTSNTLFLRIPLDESAIVVKIQGYFDAWQALLIKPDIFFKISWLYKKYEKSVKDLKDAIEVLIAEKRRRISTEEKLEECMDFATELILAEKRGDLTRENVNQCILEMLIAAPDTMSVSLFFMLFLIAKHPNVEEAIIKEIQTVIGERDIKIDDIQKLKVMENFIYESMRYQPVVDLVMRKALEDDVIDGYPVKKGTNIILNIGRMHRLEFFPKPNEFTLENFAKNVPYRYFQPFGFGPRGCAGKYIAMVMMKAILVTLLRRFHVKTLQGQCVESIQKIHDLSLHPDETKNMLEMIFTPRNSDRCLEH
Inhibitor
Name:
BDBM8871
Synonyms:
5-[(1E)-1,2,3,4-tetrahydronaphthalen-1-ylidenemethyl]-1H-imidazole | 5-[(E)-3,4-Dihydronaphthalen-1(2H)-ylidenemethyl]-1H-imidazole | Imidazolylmethylenetetrahydronaphthalene 1a | Imidazolylmethylenetetrahydronaphthalene 1b
Type:
Small organic molecule
Emp. Form.:
C14H14N2
Mol. Mass.:
210.2744
SMILES:
C1CC(=Cc2cnc[nH]2)c2ccccc2C1 |w:3.3|
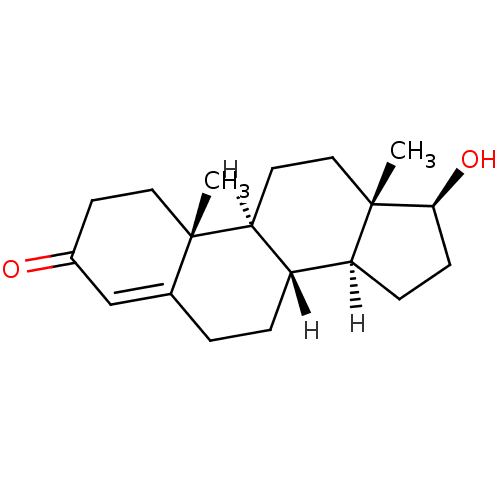
Substrate
Name:
BDBM8885
Synonyms:
(1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 17beta-Hydroxyandrost-4-en-3-one | Testosterone | Testosterone, 1 | US9682960, Testosterone
Type:
Steroid
Emp. Form.:
C19H28O2
Mol. Mass.:
288.4244
SMILES:
[H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:18|