Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 11B1, mitochondrial
Ligand
BDBM8913
Substrate
BDBM8582
Meas. Tech.
CYP11B Assay
pH
7.4±n/a
Temperature
310.15±n/a K
IC50
2724±n/a nM
Citation
 Voets, M; Antes, I; Scherer, C; Muller-Vieira, U; Biemel, K; Barassin, C; Marchais-Oberwinkler, S; Hartmann, RW Heteroaryl-substituted naphthalenes and structurally modified derivatives: selective inhibitors of CYP11B2 for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 48:6632-42 (2005) [PubMed] Article
Voets, M; Antes, I; Scherer, C; Muller-Vieira, U; Biemel, K; Barassin, C; Marchais-Oberwinkler, S; Hartmann, RW Heteroaryl-substituted naphthalenes and structurally modified derivatives: selective inhibitors of CYP11B2 for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 48:6632-42 (2005) [PubMed] Article Target
Name:
Cytochrome P450 11B1, mitochondrial
Synonyms:
C11B1_HUMAN | CYP11B1 | CYPXIB1 | Cytochrome P450 11B, mitochondrial precursor | Cytochrome P450 11B1 | Cytochrome P450 11B1 (CYP11B1) | Cytochrome P450 11B1, mitochondrial | S11BH
Type:
Enzyme
Mol. Mass.:
57591.44
Organism:
Homo sapiens (Human)
Description:
P15538
Residue:
503
Sequence:
MALRAKAEVCMAVPWLSLQRAQALGTRAARVPRTVLPFEAMPRRPGNRWLRLLQIWREQGYEDLHLEVHQTFQELGPIFRYDLGGAGMVCVMLPEDVEKLQQVDSLHPHRMSLEPWVAYRQHRGHKCGVFLLNGPEWRFNRLRLNPEVLSPNAVQRFLPMVDAVARDFSQALKKKVLQNARGSLTLDVQPSIFHYTIEASNLALFGERLGLVGHSPSSASLNFLHALEVMFKSTVQLMFMPRSLSRWTSPKVWKEHFEAWDCIFQYGDNCIQKIYQELAFSRPQQYTSIVAELLLNAELSPDAIKANSMELTAGSVDTTVFPLLMTLFELARNPNVQQALRQESLAAAASISEHPQKATTELPLLRAALKETLRLYPVGLFLERVASSDLVLQNYHIPAGTLVRVFLYSLGRNPALFPRPERYNPQRWLDIRGSGRNFYHVPFGFGMRQCLGRRLAEAEMLLLLHHVLKHLQVETLTQEDIKMVYSFILRPSMFPLLTFRAIN
Inhibitor
Name:
BDBM8913
Synonyms:
3-(1-Chloro-7-methoxy-2-naphthyl)pyridine | 3-(1-chloro-7-methoxynaphthalen-2-yl)pyridine | Pyridine-substituted naphthalene 14 | US9271963, 14
Type:
Small organic molecule
Emp. Form.:
C16H12ClNO
Mol. Mass.:
269.726
SMILES:
COc1ccc2ccc(-c3cccnc3)c(Cl)c2c1
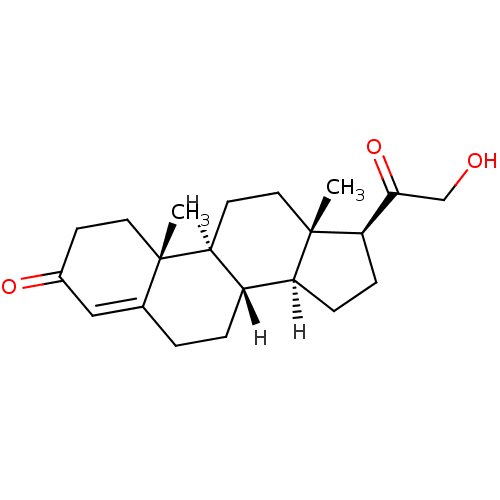
Substrate
Name:
BDBM8582
Synonyms:
(1S,2R,10S,11S,14S,15S)-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadec-6-en-5-one | 11-deoxycorticosterone | 21-hydroxypregn-4-ene-3,20-dione | DOC | [4-14C]-11-deoxycorticosterone | deoxycorticosterone
Type:
Small organic molecule
Emp. Form.:
C21H30O3
Mol. Mass.:
330.4611
SMILES:
[H][C@@]12CC[C@H](C(=O)CO)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CCC2=CC(=O)CC[C@]12C |r,t:21|