Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Genome polyprotein
Ligand
BDBM9931
Substrate
NS3-NS4A Chromogenic Peptide Substrate
Meas. Tech.
Continuous Spectrophotometric Assay
pH
6.5±n/a
Temperature
303.15±n/a K
Ki
50±n/a nM
Citation
 Bogen, S; Saksena, AK; Arasappan, A; Gu, H; Njoroge, FG; Girijavallabhan, V; Pichardo, J; Butkiewicz, N; Prongay, A; Madison, V Hepatitis C virus NS3-4A serine protease inhibitors: use of a P2-P1 cyclopropyl alanine combination for improved potency. Bioorg Med Chem Lett 15:4515-9 (2005) [PubMed] Article
Bogen, S; Saksena, AK; Arasappan, A; Gu, H; Njoroge, FG; Girijavallabhan, V; Pichardo, J; Butkiewicz, N; Prongay, A; Madison, V Hepatitis C virus NS3-4A serine protease inhibitors: use of a P2-P1 cyclopropyl alanine combination for improved potency. Bioorg Med Chem Lett 15:4515-9 (2005) [PubMed] Article More Info.:
Target
Name:
Genome polyprotein
Synonyms:
Dipeptidyl peptidase 4 (DPP4) | HCV NS3-NS4A Serine Proteinase | NS3 NS5B | NS3 Protease | NS3-4A Protease | NS3/4A Protein | NS3/4A protease | NS5B Polymerase | POLG_HCV77 | RNA polymerase (NS5B)
Type:
Protease Domain
Mol. Mass.:
327221.49
Organism:
Hepatitis C virus (HCV genotype 1a, isolate H)
Description:
P27958
Residue:
3011
Sequence:
MSTNPKPQRKTKRNTNRRPQDVKFPGGGQIVGGVYLLPRRGPRLGVRATRKTSERSQPRGRRQPIPKARRPEGRTWAQPGYPWPLYGNEGCGWAGWLLSPRGSRPSWGPTDPRRRSRNLGKVIDTLTCGFADLMGYIPLVGAPLGGAARALAHGVRVLEDGVNYATGNLPGCSFSIFLLALLSCLTVPASAYQVRNSSGLYHVTNDCPNSSVVYEAADAILHTPGCVPCVREGNASRCWVAVTPTVATRDGKLPTTQLRRHIDLLVGSATLCSALYVGDLCGSVFLVGQLFTFSPRHHWTTQDCNCSIYPGHITGHRMAWNMMMNWSPTAALVVAQLLRIPQAIMDMIAGAHWGVLAGIKYFSMVGNWAKVLVVLLLFAGVDAETHVTGGNAGRTTAGLVGLLTPGAKQNIQLINTNGSWHINSTALNCNESLNTGWLAGLFYQHKFNSSGCPERLASCRRLTDFAQGWGPISYANGSGLDERPYCWHYPPRPCGIVPAKSVCGPVYCFTPSPVVVGTTDRSGAPTYSWGANDTDVFVLNNTRPPLGNWFGCTWMNSTGFTKVCGAPPCVIGGVGNNTLLCPTDCFRKYPEATYSRCGSGPRITPRCMVDYPYRLWHYPCTINYTIFKVRMYVGGVEHRLEAACNWTRGERCDLEDRDRSELSPLLLSTTQWQVLPCSFTTLPALSTGLIHLHQNIVDVQYLYGVGSSIASWAIKWEYVVLLFLLLADARVCSCLWMMLLISQAEAALENLVILNAASLAGTHGLVSFLVFFCFAWYLKGRWVPGAVYALYGMWPLLLLLLALPQRAYALDTEVAASCGGVVLVGLMALTLSPYYKRYISWCMWWLQYFLTRVEAQLHVWVPPLNVRGGRDAVILLTCVVHPALVFDITKLLLAIFGPLWILQASLLKVPYFVRVQGLLRICALARKIAGGHYVQMAIIKLGALTGTCVYNHLAPLRDWAHNGLRDLAVAVEPVVFSRMETKLITWGADTAACGDIINGLPVSARRGQEILLGPADGMVSKGWRLLAPITAYAQQTRGLLGCIITSLTGRDKNQVEGEVQIVSTATQTFLATCINGVCWTVYHGAGTRTIASPKGPVIQTYTNVDQDLVGWPAPQGSRSLTPCTCGSSDLYLVTRHADVIPVRRRGDSRGSLLSPRPISYLKGSSGGPLLCPTGHAVGLFRAAVCTRGVAKAVDFIPVENLETTMRSPVFTDNSSPPAVPQSFQVAHLHAPTGSGKSTKVPAAYAAKGYKVLVLNPSVAATLGFGAYMSKAHGVDPNIRTGVRTITTGSPITYSTYGKFLADAGCSGGAYDIIICDECHSTDATSISGIGTVLDQAETAGARLVVLATATPPGSVTVSHPNIEEVALSTTGEIPFYGKAIPLEVIKGGRHLIFCHSKKKCDELAAKLVALGINAVAYYRGLDVSVIPTSGDVVVVSTDALMTGFTGDFDSVIDCNTCVTQTVDFSLDPTFTIETTTLPQDAVSRTQRRGRTGRGKPGIYRFVAPGERPSGMFDSSVLCECYDAGCAWYELTPAETTVRLRAYMNTPGLPVCQDHLGFWEGVFTGLTHIDAHFLSQTKQSGENFPYLVAYQATVCARAQAPPPSWDQMRKCLIRLKPTLHGPTPLLYRLGAVQNEVTLTHPITKYIMTCMSADLEVVTSTWVLVGGVLAALAAYCLSTGCVVIVGRIVLSGKPAIIPDREVLYQEFDEMEECSQHLPYIEQGMMLAEQFKQKALGLLQTASRHAEVITPAVQTNWQKLEVFWAKHMWNFISGIQYLAGLSTLPGNPAIASLMAFTAAVTSPLTTGQTLLFNILGGWVAAQLAAPGAATAFVGAGLAGAALDSVGLGKVLVDILAGYGAGVAGALVAFKIMSGEVPSTEDLVNLLPAILSPGALAVGVVFASILRRRVGPGEGAVQWMNRLIAFASRGNHVSPTHYVPESDAAARVTAILSSLTVTQLLRRLHQWISSECTTPCSGSWLRDIWDWICEVLSDFKTWLKAKLMPQLPGIPFVSCQRGYRGVWRGDGIMHTRCHCGAEITGHVKNGTMRIVGPRTCKNMWSGTFFINAYTTGPCTPLPAPNYKFALWRVSAEEYVEIRRVGDFHYVSGMTTDNLKCPCQIPSPEFFTELDGVRLHRFAPPCKPLLREEVSFRVGLHEYPVGSQLPCEPEPDVAVLTSMLTDPSHITAEAAGRRLARGSPPSMASSSASQLSAPSLKATCTANHDSPDAELIEANLLWRQEMGGNITRVESENKVVILDSFDPLVAEEDEREVSVPAEILRKSRRFAPALPVWARPDYNPLLVETWKKPDYEPPVVHGCPLPPPRSPPVPPPRKKRTVVLTESTLPTALAELATKSFGSSSTSGITGDNTTTSSEPAPSGCPPDSDVESYSSMPPLEGEPGDPDLSDGSWSTVSSGADTEDVVCCSMSYSWTGALVTPCAAEEQKLPINALSNSLLRHHNLVYSTTSRSACQRKKKVTFDRLQVLDSHYQDVLKEVKAAASKVKANLLSVEEACSLAPPHSAKSKFGYGAKDVRCHARKAVAHINSVWKDLLEDSVTPIDTTIMAKNEVFCVQPEKGGRKPARLIVFPDLGVRVCEKMALYDVVSKLPLAVMGSSYGFQYSPGQRVEFLVQAWKSKKTPMGLSYDTRCFDSTVTESDIRTEEAIYQCCDLDPQARVAIKSLTERLYVGGPLTNSRGENCGYRRCRASRVLTTSCGNTLTRYIKARAACRAAGLQDCTMLVCGDDLVVICESAGVQEDAASLRAFTEAMTRYSAPPGDPPQPEYDLELITSCSSNVSVAHDGAGKRVYYLTRDPTTPLARAAWETARHTPVNSWLGNIIMFAPTLWARMILMTHFFSVLIARDQLEQALNCEIYGACYSIEPLDLPPIIQRLHGLSAFSLHSYSPGEINRVAACLRKLGVPPLRAWRHRAWSVRARLLARGGKAAICGKYLFNWAVRTKLKLTPITAAGRLDLSGWFTAGYSGGDIYHSVSHARPRWFWFCLLLLAAGVGIYLLPNR
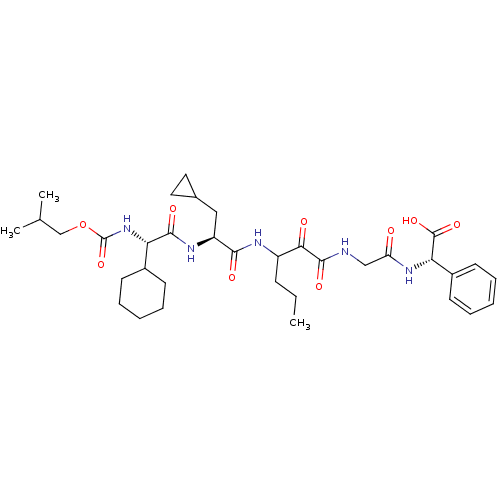
Inhibitor
Name:
BDBM9931
Synonyms:
(2S)-2-(2-{3-[(2S)-2-[(2S)-2-cyclohexyl-2-{[(2-methylpropoxy)carbonyl]amino}acetamido]-3-cyclopropylpropanamido]-2-oxohexanamido}acetamido)-2-phenylacetic acid | P2 side-chain modified alpha-ketoamide inhibitor 23
Type:
Small organic molecule
Emp. Form.:
C35H51N5O9
Mol. Mass.:
685.8075
SMILES:
CCCC(NC(=O)[C@H](CC1CC1)NC(=O)[C@@H](NC(=O)OCC(C)C)C1CCCCC1)C(=O)C(=O)NCC(=O)N[C@H](C(O)=O)c1ccccc1 |r|
Substrate
Name:
NS3-NS4A Chromogenic Peptide Substrate
Synonyms:
n/a
Type:
Peptide
Mol. Mass.:
1366.94
Organism:
n/a
Description:
The 4-phenylazophenol (PAP) chromophore was incorporated to improve assay sensitivity.
Residue:
13
Sequence:
ACDTEDVVPNVAH