Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Stromelysin-1
Ligand
BDBM11874
Substrate
MMP-3 Fluorogenic Peptide Substrate
Meas. Tech.
Enzyme Inhibition Assay
Ki
0.3±n/a nM
Citation
 Becker, DP; Villamil, CI; Barta, TE; Bedell, LJ; Boehm, TL; Decrescenzo, GA; Freskos, JN; Getman, DP; Hockerman, S; Heintz, R; Howard, SC; Li, MH; McDonald, JJ; Carron, CP; Funckes-Shippy, CL; Mehta, PP; Munie, GE; Swearingen, CA Synthesis and structure-activity relationships of beta- and alpha-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J Med Chem 48:6713-30 (2005) [PubMed] Article
Becker, DP; Villamil, CI; Barta, TE; Bedell, LJ; Boehm, TL; Decrescenzo, GA; Freskos, JN; Getman, DP; Hockerman, S; Heintz, R; Howard, SC; Li, MH; McDonald, JJ; Carron, CP; Funckes-Shippy, CL; Mehta, PP; Munie, GE; Swearingen, CA Synthesis and structure-activity relationships of beta- and alpha-piperidine sulfone hydroxamic acid matrix metalloproteinase inhibitors with oral antitumor efficacy. J Med Chem 48:6713-30 (2005) [PubMed] Article More Info.:
Target
Name:
Stromelysin-1
Synonyms:
MMP-3 | MMP3 | MMP3_HUMAN | Matrix metalloproteinase (2 and 3) | Matrix metalloproteinase 3 | Matrix metalloproteinase-3 | Matrix metalloproteinase-3 (MMP-3) | Matrix metalloproteinase-3 (MMP3) | SL-1 | STMY1 | Stromelysin 1 | Transin-1
Type:
Enzyme
Mol. Mass.:
53973.13
Organism:
Homo sapiens (Human)
Description:
P08254
Residue:
477
Sequence:
MKSLPILLLLCVAVCSAYPLDGAARGEDTSMNLVQKYLENYYDLKKDVKQFVRRKDSGPVVKKIREMQKFLGLEVTGKLDSDTLEVMRKPRCGVPDVGHFRTFPGIPKWRKTHLTYRIVNYTPDLPKDAVDSAVEKALKVWEEVTPLTFSRLYEGEADIMISFAVREHGDFYPFDGPGNVLAHAYAPGPGINGDAHFDDDEQWTKDTTGTNLFLVAAHEIGHSLGLFHSANTEALMYPLYHSLTDLTRFRLSQDDINGIQSLYGPPPDSPETPLVPTEPVPPEPGTPANCDPALSFDAVSTLRGEILIFKDRHFWRKSLRKLEPELHLISSFWPSLPSGVDAAYEVTSKDLVFIFKGNQFWAIRGNEVRAGYPRGIHTLGFPPTVRKIDAAISDKEKNKTYFFVEDKYWRFDEKRNSMEPGFPKQIAEDFPGIDSKIDAVFEEFGFFYFFTGSSQLEFDPNAKKVTHTLKSNSWLNC
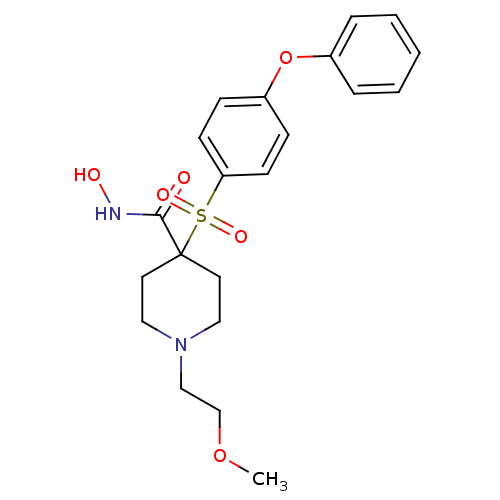
Inhibitor
Name:
BDBM11874
Synonyms:
N-Hydroxy-1-(2-methoxyethyl)-4-{[4-(phenoxyphenyl]-sulfonyl}piperidine-4-carboxamide Hydrochloride | N-hydroxy-1-(2-methoxyethyl)-4-[(4-phenoxybenzene)sulfonyl]piperidine-4-carboxamide hydrochloride | alpha-sulfone 27d
Type:
Small organic molecule
Emp. Form.:
C21H26N2O6S
Mol. Mass.:
434.506
SMILES:
COCCN1CCC(CC1)(C(=O)NO)S(=O)(=O)c1ccc(Oc2ccccc2)cc1
Substrate
Name:
MMP-3 Fluorogenic Peptide Substrate
Synonyms:
NFF-3
Type:
Peptide
Mol. Mass.:
3799.85
Organism:
n/a
Description:
n/a
Residue:
35
Sequence:
MCAARGPRLYSPRVALGLNVATRPARGLYSDNPNH