Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Dipeptidyl peptidase 9
Ligand
BDBM12652
Substrate
BDBM11057
Meas. Tech.
DPP Inhibition Assay
pH
7.5±n/a
Temperature
295.15±n/a K
Ki
73±n/a nM
Comments
Result is from racemic analogues.
Citation
 Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 49:6439-42 (2006) [PubMed] Article
Pei, Z; Li, X; von Geldern, TW; Madar, DJ; Longenecker, K; Yong, H; Lubben, TH; Stewart, KD; Zinker, BA; Backes, BJ; Judd, AS; Mulhern, M; Ballaron, SJ; Stashko, MA; Mika, AK; Beno, DW; Reinhart, GA; Fryer, RM; Preusser, LC; Kempf-Grote, AJ; Sham, HL; Trevillyan, JM Discovery of ((4R,5S)-5-amino-4-(2,4,5- trifluorophenyl)cyclohex-1-enyl)-(3- (trifluoromethyl)-5,6-dihydro- [1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl)methanone (ABT-341), a highly potent, selective, orally efficacious, and safe dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 49:6439-42 (2006) [PubMed] Article More Info.:
Target
Name:
Dipeptidyl peptidase 9
Synonyms:
DPP9 | DPP9_HUMAN | DPRP-2 | DPRP2 | Dipeptidyl peptidase 9 (DDP9) | Dipeptidyl peptidase 9 (DPP-9) | Dipeptidyl peptidase 9 (DPP9) | Dipeptidyl peptidase IV-related protein 2 | Dipeptidyl peptidase IX | Dipeptidyl peptidase IX (DDP-IX) | Dipeptidyl peptidase-like protein 9
Type:
Enzyme
Mol. Mass.:
98260.70
Organism:
Homo sapiens (Human)
Description:
Q86TI2
Residue:
863
Sequence:
MATTGTPTADRGDAAATDDPAARFQVQKHSWDGLRSIIHGSRKYSGLIVNKAPHDFQFVQKTDESGPHSHRLYYLGMPYGSRENSLLYSEIPKKVRKEALLLLSWKQMLDHFQATPHHGVYSREEELLRERKRLGVFGITSYDFHSESGLFLFQASNSLFHCRDGGKNGFMVSPMKPLEIKTQCSGPRMDPKICPADPAFFSFINNSDLWVANIETGEERRLTFCHQGLSNVLDDPKSAGVATFVIQEEFDRFTGYWWCPTASWEGSEGLKTLRILYEEVDESEVEVIHVPSPALEERKTDSYRYPRTGSKNPKIALKLAEFQTDSQGKIVSTQEKELVQPFSSLFPKVEYIARAGWTRDGKYAWAMFLDRPQQWLQLVLLPPALFIPSTENEEQRLASARAVPRNVQPYVVYEEVTNVWINVHDIFYPFPQSEGEDELCFLRANECKTGFCHLYKVTAVLKSQGYDWSEPFSPGEDEFKCPIKEEIALTSGEWEVLARHGSKIWVNEETKLVYFQGTKDTPLEHHLYVVSYEAAGEIVRLTTPGFSHSCSMSQNFDMFVSHYSSVSTPPCVHVYKLSGPDDDPLHKQPRFWASMMEAASCPPDYVPPEIFHFHTRSDVRLYGMIYKPHALQPGKKHPTVLFVYGGPQVQLVNNSFKGIKYLRLNTLASLGYAVVVIDGRGSCQRGLRFEGALKNQMGQVEIEDQVEGLQFVAEKYGFIDLSRVAIHGWSYGGFLSLMGLIHKPQVFKVAIAGAPVTVWMAYDTGYTERYMDVPENNQHGYEAGSVALHVEKLPNEPNRLLILHGFLDENVHFFHTNFLVSQLIRAGKPYQLQIYPNERHSIRCPESGEHYEVTLLHFLQEYL
Inhibitor
Name:
BDBM12652
Synonyms:
(1S,6R)-3-(1,2,3,4-tetrahydroisoquinolin-2-ylmethyl)-6-(2,4,5-trifluorophenyl)cyclohex-3-en-1-aminium; 2,2,2-trifluoroacetate | ABT-341 analogue 14
Type:
Small organic molecule
Emp. Form.:
C22H24F3N2
Mol. Mass.:
373.434
SMILES:
[NH3+][C@H]1CC(CN2CCc3ccccc3C2)=CC[C@@H]1c1cc(F)c(F)cc1F |r,c:16|
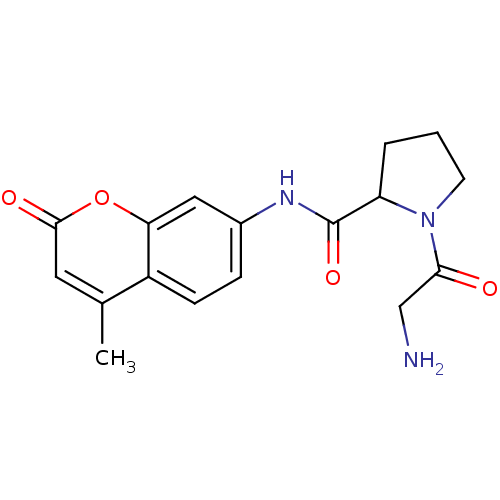
Substrate
Name:
BDBM11057
Synonyms:
1-(2-aminoacetyl)-N-(4-methyl-2-oxo-2H-chromen-7-yl)pyrrolidine-2-carboxamide hydrobromide | Gly-Pro-7-amido-4-methylcoumarin hydrobromide | Gly-Pro-7-amidomethylcoumarin | Gly-Pro-AMC
Type:
Small organic molecule
Emp. Form.:
C17H19N3O4
Mol. Mass.:
329.3505
SMILES:
Cc1cc(=O)oc2cc(NC(=O)C3CCCN3C(=O)CN)ccc12