Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Serine/threonine-protein kinase ATR
Ligand
BDBM350085
Substrate
n/a
Meas. Tech.
ATR Inhibition Assay
Ki
<1.000±n/a nM
Citation
 Charrier, J; Studley, J; Pierard, FY; Durrant, SJ; Littler, BJ; Hughes, RM; Siesel, DA; Angell, P; Urbina, A; Shi, Y Processes for preparing ATR inhibitors US Patent US10208027 Publication Date 2/19/2019
Charrier, J; Studley, J; Pierard, FY; Durrant, SJ; Littler, BJ; Hughes, RM; Siesel, DA; Angell, P; Urbina, A; Shi, Y Processes for preparing ATR inhibitors US Patent US10208027 Publication Date 2/19/2019 More Info.:
Target
Name:
Serine/threonine-protein kinase ATR
Synonyms:
ATR | ATR_HUMAN | Ataxia telangiectasia and Rad3-related protein (ATR) | FRAP-related protein 1 | FRP1 | Serine-protein kinase ATR
Type:
Protein
Mol. Mass.:
301404.58
Organism:
Homo sapiens (Human)
Description:
Q13535
Residue:
2644
Sequence:
MGEHGLELASMIPALRELGSATPEEYNTVVQKPRQILCQFIDRILTDVNVVAVELVKKTDSQPTSVMLLDFIQHIMKSSPLMFVNVSGSHEAKGSCIEFSNWIITRLLRIAATPSCHLLHKKICEVICSLLFLFKSKSPAIFGVLTKELLQLFEDLVYLHRRNVMGHAVEWPVVMSRFLSQLDEHMGYLQSAPLQLMSMQNLEFIEVTLLMVLTRIIAIVFFRRQELLLWQIGCVLLEYGSPKIKSLAISFLTELFQLGGLPAQPASTFFSSFLELLKHLVEMDTDQLKLYEEPLSKLIKTLFPFEAEAYRNIEPVYLNMLLEKLCVMFEDGVLMRLKSDLLKAALCHLLQYFLKFVPAGYESALQVRKVYVRNICKALLDVLGIEVDAEYLLGPLYAALKMESMEIIEEIQCQTQQENLSSNSDGISPKRRRLSSSLNPSKRAPKQTEEIKHVDMNQKSILWSALKQKAESLQISLEYSGLKNPVIEMLEGIAVVLQLTALCTVHCSHQNMNCRTFKDCQHKSKKKPSVVITWMSLDFYTKVLKSCRSLLESVQKLDLEATIDKVVKIYDALIYMQVNSSFEDHILEDLCGMLSLPWIYSHSDDGCLKLTTFAANLLTLSCRISDSYSPQAQSRCVFLLTLFPRRIFLEWRTAVYNWALQSSHEVIRASCVSGFFILLQQQNSCNRVPKILIDKVKDDSDIVKKEFASILGQLVCTLHGMFYLTSSLTEPFSEHGHVDLFCRNLKATSQHECSSSQLKASVCKPFLFLLKKKIPSPVKLAFIDNLHHLCKHLDFREDETDVKAVLGTLLNLMEDPDKDVRVAFSGNIKHILESLDSEDGFIKELFVLRMKEAYTHAQISRNNELKDTLILTTGDIGRAAKGDLVPFALLHLLHCLLSKSASVSGAAYTEIRALVAAKSVKLQSFFSQYKKPICQFLVESLHSSQMTALPNTPCQNADVRKQDVAHQREMALNTLSEIANVFDFPDLNRFLTRTLQVLLPDLAAKASPAASALIRTLGKQLNVNRREILINNFKYIFSHLVCSCSKDELERALHYLKNETEIELGSLLRQDFQGLHNELLLRIGEHYQQVFNGLSILASFASSDDPYQGPRDIISPELMADYLQPKLLGILAFFNMQLLSSSVGIEDKKMALNSLMSLMKLMGPKHVSSVRVKMMTTLRTGLRFKDDFPELCCRAWDCFVRCLDHACLGSLLSHVIVALLPLIHIQPKETAAIFHYLIIENRDAVQDFLHEIYFLPDHPELKKIKAVLQEYRKETSESTDLQTTLQLSMKAIQHENVDVRIHALTSLKETLYKNQEKLIKYATDSETVEPIISQLVTVLLKGCQDANSQARLLCGECLGELGAIDPGRLDFSTTETQGKDFTFVTGVEDSSFAYGLLMELTRAYLAYADNSRAQDSAAYAIQELLSIYDCREMETNGPGHQLWRRFPEHVREILEPHLNTRYKSSQKSTDWSGVKKPIYLSKLGSNFAEWSASWAGYLITKVRHDLASKIFTCCSIMMKHDFKVTIYLLPHILVYVLLGCNQEDQQEVYAEIMAVLKHDDQHTINTQDIASDLCQLSTQTVFSMLDHLTQWARHKFQALKAEKCPHSKSNRNKVDSMVSTVDYEDYQSVTRFLDLIPQDTLAVASFRSKAYTRAVMHFESFITEKKQNIQEHLGFLQKLYAAMHEPDGVAGVSAIRKAEPSLKEQILEHESLGLLRDATACYDRAIQLEPDQIIHYHGVVKSMLGLGQLSTVITQVNGVHANRSEWTDELNTYRVEAAWKLSQWDLVENYLAADGKSTTWSVRLGQLLLSAKKRDITAFYDSLKLVRAEQIVPLSAASFERGSYQRGYEYIVRLHMLCELEHSIKPLFQHSPGDSSQEDSLNWVARLEMTQNSYRAKEPILALRRALLSLNKRPDYNEMVGECWLQSARVARKAGHHQTAYNALLNAGESRLAELYVERAKWLWSKGDVHQALIVLQKGVELCFPENETPPEGKNMLIHGRAMLLVGRFMEETANFESNAIMKKYKDVTACLPEWEDGHFYLAKYYDKLMPMVTDNKMEKQGDLIRYIVLHFGRSLQYGNQFIYQSMPRMLTLWLDYGTKAYEWEKAGRSDRVQMRNDLGKINKVITEHTNYLAPYQFLTAFSQLISRICHSHDEVFVVLMEIIAKVFLAYPQQAMWMMTAVSKSSYPMRVNRCKEILNKAIHMKKSLEKFVGDATRLTDKLLELCNKPVDGSSSTLSMSTHFKMLKKLVEEATFSEILIPLQSVMIPTLPSILGTHANHASHEPFPGHWAYIAGFDDMVEILASLQKPKKISLKGSDGKFYIMMCKPKDDLRKDCRLMEFNSLINKCLRKDAESRRRELHIRTYAVIPLNDECGIIEWVNNTAGLRPILTKLYKEKGVYMTGKELRQCMLPKSAALSEKLKVFREFLLPRHPPIFHEWFLRTFPDPTSWYSSRSAYCRSTAVMSMVGYILGLGDRHGENILFDSLTGECVHVDFNCLFNKGETFEVPEIVPFRLTHNMVNGMGPMGTEGLFRRACEVTMRLMRDQREPLMSVLKTFLHDPLVEWSKPVKGHSKAPLNETGEVVNEKAKTHVLDIEQRLQGVIKTRNRVTGLPLSIEGHVHYLIQEATDENLLCQMYLGWTPYM
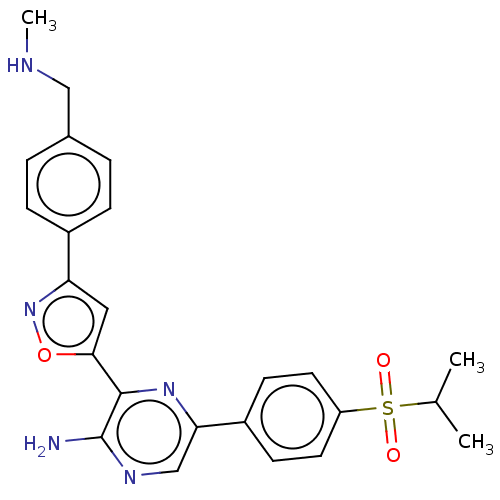
Inhibitor
Name:
BDBM350085
Synonyms:
3-[3-[4-[dideuterio(methylamino)methyl]phenyl]isoxazol-5-yl]-5-(4-isopropylsulfonylphenyl)pyrazin-2-amine | BDBM50226746 | US10208027, Compound II-1 | US10208027, Compound II-2 | US10208027, Compound II-3 | US10208027, Compound II-4 | US10479784, Compound IIA-7 | US10822331, Cmpd II-4 | US10961232, Compound IIA-7 | US11787781, Compound A
Type:
Small organic molecule
Emp. Form.:
C24H25N5O3S
Mol. Mass.:
463.552
SMILES:
CNCc1ccc(cc1)-c1cc(on1)-c1nc(cnc1N)-c1ccc(cc1)S(=O)(=O)C(C)C