Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50043697
Substrate
n/a
Meas. Tech.
hERG Activity Assays
IC50
>30000±n/a nM
Citation
 Xu, Y; Brenning, BG; Kultgen, SG; Liu, X; Saunders, MD; Ho, K Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitors US Patent US10047093 Publication Date 8/14/2018
Xu, Y; Brenning, BG; Kultgen, SG; Liu, X; Saunders, MD; Ho, K Substituted imidazo[1,2-B]pyridazines as protein kinase inhibitors US Patent US10047093 Publication Date 8/14/2018 More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
Name:
BDBM50043697
Synonyms:
CHEMBL3355987 | US10047093, 8-63 | US10392392, Example 8-63 | US10875864, EX. 8-63 | US9416132, 8-63
Type:
Small organic molecule
Emp. Form.:
C21H25ClN4O
Mol. Mass.:
384.902
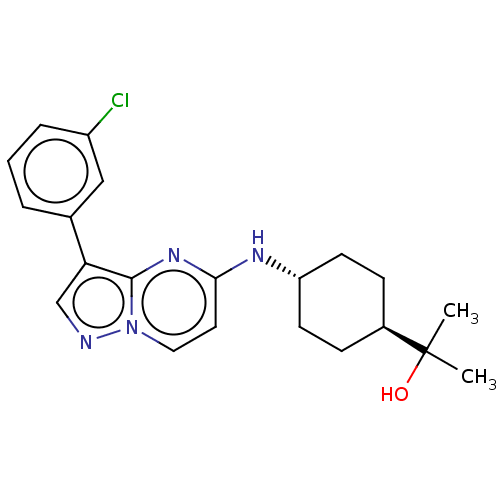
SMILES:
CC(C)(O)[C@H]1CC[C@@H](CC1)Nc1ccn2ncc(-c3cccc(Cl)c3)c2n1 |r,wU:4.3,wD:7.10,(.73,-52.37,;1.51,-51.04,;-.05,-51.03,;1.52,-49.51,;2.84,-51.82,;4.18,-51.06,;5.51,-51.82,;5.5,-53.37,;4.18,-54.14,;2.84,-53.36,;6.84,-54.14,;8.17,-53.37,;8.17,-51.83,;9.5,-51.05,;10.83,-51.83,;12.29,-51.35,;13.2,-52.6,;12.29,-53.84,;13.05,-55.17,;12.26,-56.5,;13.02,-57.84,;14.56,-57.86,;15.34,-56.52,;16.88,-56.53,;14.58,-55.19,;10.83,-53.37,;9.5,-54.13,)|