Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 2D6
Ligand
BDBM21358
Substrate
BDBM21362
Meas. Tech.
Cytochrome P450 Inhibition
pH
7.4±n/a
Temperature
310.15±n/a K
IC50
196000±n/a nM
Citation
 Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem 50:5535-8 (2007) [PubMed] Article
Cole, DC; Stock, JR; Lennox, WJ; Bernotas, RC; Ellingboe, JW; Boikess, S; Coupet, J; Smith, DL; Leung, L; Zhang, GM; Feng, X; Kelly, MF; Galante, R; Huang, P; Dawson, LA; Marquis, K; Rosenzweig-Lipson, S; Beyer, CE; Schechter, LE Discovery of N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine as a potent, selective, and orally active 5-HT(6) receptor agonist. J Med Chem 50:5535-8 (2007) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 2D6
Synonyms:
CP2D6_HUMAN | CYP2D6 | CYP2DL1 | CYPIID6 | Cytochrome P450 2D6 (CYP2D6) | Debrisoquine 4-hydroxylase | P450-DB1
Type:
Protein
Mol. Mass.:
55774.82
Organism:
Homo sapiens (Human)
Description:
P10635
Residue:
497
Sequence:
MGLEALVPLAVIVAIFLLLVDLMHRRQRWAARYPPGPLPLPGLGNLLHVDFQNTPYCFDQLRRRFGDVFSLQLAWTPVVVLNGLAAVREALVTHGEDTADRPPVPITQILGFGPRSQGVFLARYGPAWREQRRFSVSTLRNLGLGKKSLEQWVTEEAACLCAAFANHSGRPFRPNGLLDKAVSNVIASLTCGRRFEYDDPRFLRLLDLAQEGLKEESGFLREVLNAVPVLLHIPALAGKVLRFQKAFLTQLDELLTEHRMTWDPAQPPRDLTEAFLAEMEKAKGNPESSFNDENLRIVVADLFSAGMVTTSTTLAWGLLLMILHPDVQRRVQQEIDDVIGQVRRPEMGDQAHMPYTTAVIHEVQRFGDIVPLGVTHMTSRDIEVQGFRIPKGTTLITNLSSVLKDEAVWEKPFRFHPEHFLDAQGHFVKPEAFLPFSAGRRACLGEPLARMELFLFFTSLLQHFSFSVPTGQPRPSHHGVFAFLVSPSPYELCAVPR
Inhibitor
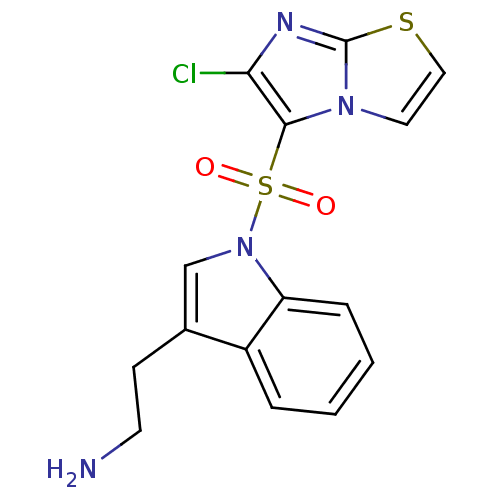
Name:
BDBM21358
Synonyms:
2-[1-({6-chloroimidazo[2,1-b][1,3]thiazole-5-}sulfonyl)-1H-indol-3-yl]ethan-1-amine | N1-(6-chloroimidazo[2,1-b][1,3]thiazole-5-sulfonyl)tryptamine | SAX-187 | WAY-181187
Type:
Small organic molecule
Emp. Form.:
C15H13ClN4O2S2
Mol. Mass.:
380.872
SMILES:
NCCc1cn(c2ccccc12)S(=O)(=O)c1c(Cl)nc2sccn12