Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sodium channel protein type 5 subunit alpha
Ligand
BDBM372445
Substrate
n/a
Meas. Tech.
In Vitro Measure of Sodium Channel Blocking Activity and Reversibility
IC50
8.80±n/a nM
Citation
 Johnson, MR 3,5-diamino-6-chloro-N-(N-(4-phenylbutyl)carbamimidoyl) pyrazine-2-carboxamide compounds US Patent US10246425 Publication Date 4/2/2019
Johnson, MR 3,5-diamino-6-chloro-N-(N-(4-phenylbutyl)carbamimidoyl) pyrazine-2-carboxamide compounds US Patent US10246425 Publication Date 4/2/2019 More Info.:
Target
Name:
Sodium channel protein type 5 subunit alpha
Synonyms:
HH1 | SCN5A | SCN5A_HUMAN | Sodium channel alpha subunit | Sodium channel protein type 5 subunit alpha/beta-1/beta-2 | Sodium channel protein type V alpha subunit | Voltage-gated sodium channel subunit alpha Nav1.5
Type:
Enzyme
Mol. Mass.:
226908.60
Organism:
Homo sapiens (Human)
Description:
Q14524
Residue:
2016
Sequence:
MANFLLPRGTSSFRRFTRESLAAIEKRMAEKQARGSTTLQESREGLPEEEAPRPQLDLQASKKLPDLYGNPPQELIGEPLEDLDPFYSTQKTFIVLNKGKTIFRFSATNALYVLSPFHPIRRAAVKILVHSLFNMLIMCTILTNCVFMAQHDPPPWTKYVEYTFTAIYTFESLVKILARGFCLHAFTFLRDPWNWLDFSVIIMAYTTEFVDLGNVSALRTFRVLRALKTISVISGLKTIVGALIQSVKKLADVMVLTVFCLSVFALIGLQLFMGNLRHKCVRNFTALNGTNGSVEADGLVWESLDLYLSDPENYLLKNGTSDVLLCGNSSDAGTCPEGYRCLKAGENPDHGYTSFDSFAWAFLALFRLMTQDCWERLYQQTLRSAGKIYMIFFMLVIFLGSFYLVNLILAVVAMAYEEQNQATIAETEEKEKRFQEAMEMLKKEHEALTIRGVDTVSRSSLEMSPLAPVNSHERRSKRRKRMSSGTEECGEDRLPKSDSEDGPRAMNHLSLTRGLSRTSMKPRSSRGSIFTFRRRDLGSEADFADDENSTAGESESHHTSLLVPWPLRRTSAQGQPSPGTSAPGHALHGKKNSTVDCNGVVSLLGAGDPEATSPGSHLLRPVMLEHPPDTTTPSEEPGGPQMLTSQAPCVDGFEEPGARQRALSAVSVLTSALEELEESRHKCPPCWNRLAQRYLIWECCPLWMSIKQGVKLVVMDPFTDLTITMCIVLNTLFMALEHYNMTSEFEEMLQVGNLVFTGIFTAEMTFKIIALDPYYYFQQGWNIFDSIIVILSLMELGLSRMSNLSVLRSFRLLRVFKLAKSWPTLNTLIKIIGNSVGALGNLTLVLAIIVFIFAVVGMQLFGKNYSELRDSDSGLLPRWHMMDFFHAFLIIFRILCGEWIETMWDCMEVSGQSLCLLVFLLVMVIGNLVVLNLFLALLLSSFSADNLTAPDEDREMNNLQLALARIQRGLRFVKRTTWDFCCGLLRQRPQKPAALAAQGQLPSCIATPYSPPPPETEKVPPTRKETRFEEGEQPGQGTPGDPEPVCVPIAVAESDTDDQEEDEENSLGTEEESSKQQESQPVSGGPEAPPDSRTWSQVSATASSEAEASASQADWRQQWKAEPQAPGCGETPEDSCSEGSTADMTNTAELLEQIPDLGQDVKDPEDCFTEGCVRRCPCCAVDTTQAPGKVWWRLRKTCYHIVEHSWFETFIIFMILLSSGALAFEDIYLEERKTIKVLLEYADKMFTYVFVLEMLLKWVAYGFKKYFTNAWCWLDFLIVDVSLVSLVANTLGFAEMGPIKSLRTLRALRPLRALSRFEGMRVVVNALVGAIPSIMNVLLVCLIFWLIFSIMGVNLFAGKFGRCINQTEGDLPLNYTIVNNKSQCESLNLTGELYWTKVKVNFDNVGAGYLALLQVATFKGWMDIMYAAVDSRGYEEQPQWEYNLYMYIYFVIFIIFGSFFTLNLFIGVIIDNFNQQKKKLGGQDIFMTEEQKKYYNAMKKLGSKKPQKPIPRPLNKYQGFIFDIVTKQAFDVTIMFLICLNMVTMMVETDDQSPEKINILAKINLLFVAIFTGECIVKLAALRHYYFTNSWNIFDFVVVILSIVGTVLSDIIQKYFFSPTLFRVIRLARIGRILRLIRGAKGIRTLLFALMMSLPALFNIGLLLFLVMFIYSIFGMANFAYVKWEAGIDDMFNFQTFANSMLCLFQITTSAGWDGLLSPILNTGPPYCDPTLPNSNGSRGDCGSPAVGILFFTTYIIISFLIVVNMYIAIILENFSVATEESTEPLSEDDFDMFYEIWEKFDPEATQFIEYSVLSDFADALSEPLRIAKPNQISLINMDLPMVSGDRIHCMDILFAFTKRVLGESGEMDALKIQMEEKFMAANPSKISYEPITTTLRRKHEEVSAMVIQRAFRRHLLQRSLKHASFLFRQQAGSGLSEEDAPEREGLIAYVMSENFSRPLGPPSSSSISSTSFPPSYDSVTRATSDNLQVRGSDYSHSEDLADFPPSPDRDRESIV
Inhibitor
Name:
BDBM372445
Synonyms:
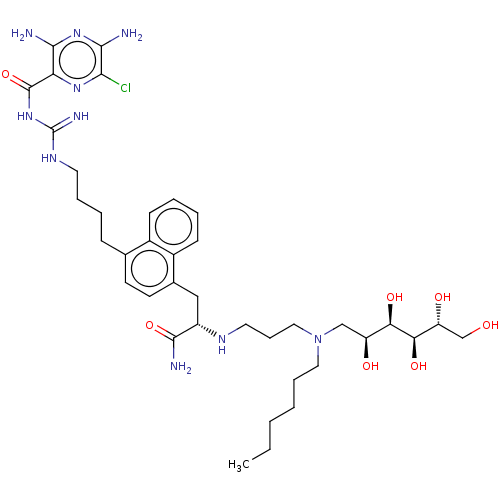
3,5-diamino-N-(N-(4-(4-((S)-3-amino-2-(3-(hexyl((2S,3R,4R,5R)-2,3,4,5,6-pentahydroxyhexyl)amino)propylamino)-3-oxopropyl)naphthalen-1-yl)butyl)carbamimidoyl)-6-chloropyrazine-2-carboxamide | US10246425, Compound VIII-d
Type:
Small organic molecule
Emp. Form.:
C38H59ClN10O7
Mol. Mass.:
803.391
SMILES:
CCCCCCN(CCCN[C@@H](Cc1ccc(CCCCNC(=N)NC(=O)c2nc(Cl)c(N)nc2N)c2ccccc12)C(N)=O)C[C@H](O)[C@@H](O)[C@H](O)[C@H](O)CO