Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glycogen phosphorylase, muscle form
Ligand
BDBM23212
Substrate
BDBM23188
Meas. Tech.
Enzyme Inhibition Assay
pH
7.2±n/a
Temperature
295.15±n/a K
Comments
No activity.
Citation
 Wen, X; Sun, H; Liu, J; Cheng, K; Zhang, P; Zhang, L; Hao, J; Zhang, L; Ni, P; Zographos, SE; Leonidas, DD; Alexacou, KM; Gimisis, T; Hayes, JM; Oikonomakos, NG Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure-Activity Relationships, and X-ray Crystallographic Studies. J Med Chem 51:3540-54 (2008) [PubMed] Article
Wen, X; Sun, H; Liu, J; Cheng, K; Zhang, P; Zhang, L; Hao, J; Zhang, L; Ni, P; Zographos, SE; Leonidas, DD; Alexacou, KM; Gimisis, T; Hayes, JM; Oikonomakos, NG Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure-Activity Relationships, and X-ray Crystallographic Studies. J Med Chem 51:3540-54 (2008) [PubMed] Article Target
Name:
Glycogen phosphorylase, muscle form
Synonyms:
Glycogen Phosphorylase (PYGM) | Glycogen phosphorylase a (RMGPa) | Glycogen phosphorylase, muscle form | Myophosphorylase | PYGM | PYGM_RABIT
Type:
Enzyme
Mol. Mass.:
97296.32
Organism:
Oryctolagus cuniculus (rabbit)
Description:
Phosphorylation of Ser-15 converts phosphorylase B (unphosphorylated) to phosphorylase A.
Residue:
843
Sequence:
MSRPLSDQEKRKQISVRGLAGVENVTELKKNFNRHLHFTLVKDRNVATPRDYYFALAHTVRDHLVGRWIRTQQHYYEKDPKRIYYLSLEFYMGRTLQNTMVNLALENACDEATYQLGLDMEELEEIEEDAGLGNGGLGRLAACFLDSMATLGLAAYGYGIRYEFGIFNQKICGGWQMEEADDWLRYGNPWEKARPEFTLPVHFYGRVEHTSQGAKWVDTQVVLAMPYDTPVPGYRNNVVNTMRLWSAKAPNDFNLKDFNVGGYIQAVLDRNLAENISRVLYPNDNFFEGKELRLKQEYFVVAATLQDIIRRFKSSKFGCRDPVRTNFDAFPDKVAIQLNDTHPSLAIPELMRVLVDLERLDWDKAWEVTVKTCAYTNHTVLPEALERWPVHLLETLLPRHLQIIYEINQRFLNRVAAAFPGDVDRLRRMSLVEEGAVKRINMAHLCIAGSHAVNGVARIHSEILKKTIFKDFYELEPHKFQNKTNGITPRRWLVLCNPGLAEIIAERIGEEYISDLDQLRKLLSYVDDEAFIRDVAKVKQENKLKFAAYLEREYKVHINPNSLFDVQVKRIHEYKRQLLNCLHVITLYNRIKKEPNKFVVPRTVMIGGKAAPGYHMAKMIIKLITAIGDVVNHDPVVGDRLRVIFLENYRVSLAEKVIPAADLSEQISTAGTEASGTGNMKFMLNGALTIGTMDGANVEMAEEAGEENFFIFGMRVEDVDRLDQRGYNAQEYYDRIPELRQIIEQLSSGFFSPKQPDLFKDIVNMLMHHDRFKVFADYEEYVKCQERVSALYKNPREWTRMVIRNIATSGKFSSDRTIAQYAREIWGVEPSRQRLPAPDEKIP
Inhibitor
Name:
BDBM23212
Synonyms:
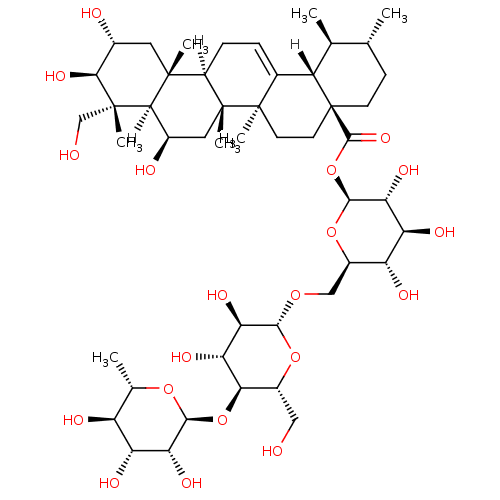
(2S,3R,4S,5S,6R)-6-({[(2R,3R,4R,5S,6R)-3,4-dihydroxy-6-(hydroxymethyl)-5-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}oxan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl (1S,2R,4aS,6aS,6bR,8R,8aR,9R,10R,11R,12aR,12bR,14bS)-8,10,11-trihydroxy-9-(hydroxymethyl)-1,2,6a,6b,9,12a-hexamethyl-1,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,12b,13,14b-icosahydropicene-4a-carboxylate | Ursane Triterpene, 22
Type:
Terpene
Emp. Form.:
C48H78O20
Mol. Mass.:
975.1209
SMILES:
[H][C@]12CC=C3[C@]4([H])[C@@H](C)[C@H](C)CC[C@@]4(CC[C@@]3(C)[C@]1(C)C[C@@H](O)[C@@]1([H])[C@](C)(CO)[C@@H](O)[C@H](O)C[C@]21C)C(=O)O[C@@H]1O[C@H](CO[C@@H]2O[C@H](CO)[C@@H](O[C@@H]3O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@@H](O)[C@H](O)[C@H]1O |t:3|