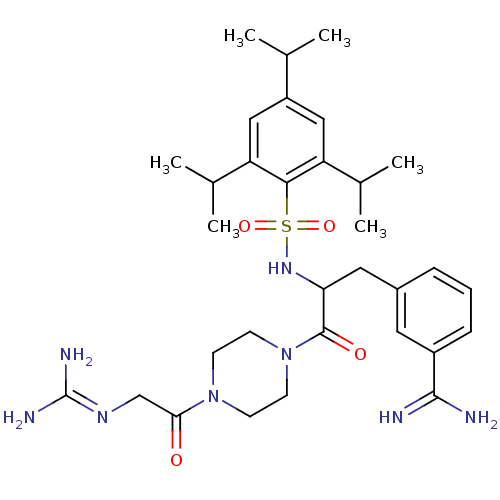
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Suppressor of tumorigenicity 14 protein
Ligand
BDBM23872
Substrate
BDBM23868
Meas. Tech.
Determination of Inhibition Constants
pH
8±n/a
Temperature
298.15±n/a K
Ki
330±n/a nM
Citation
 Steinmetzer, T; Schweinitz, A; Stürzebecher, A; Dönnecke, D; Uhland, K; Schuster, O; Steinmetzer, P; Müller, F; Friedrich, R; Than, ME; Bode, W; Stürzebecher, J Secondary amides of sulfonylated 3-amidinophenylalanine. New potent and selective inhibitors of matriptase. J Med Chem 49:4116-26 (2006) [PubMed] Article
Steinmetzer, T; Schweinitz, A; Stürzebecher, A; Dönnecke, D; Uhland, K; Schuster, O; Steinmetzer, P; Müller, F; Friedrich, R; Than, ME; Bode, W; Stürzebecher, J Secondary amides of sulfonylated 3-amidinophenylalanine. New potent and selective inhibitors of matriptase. J Med Chem 49:4116-26 (2006) [PubMed] Article More Info.:
Target
Name:
Suppressor of tumorigenicity 14 protein
Synonyms:
Epithin | Hepatocyte growth factor activator/Serine protease hepsin/Suppressor of tumorigenicity 14 protein | MT-SP1 | Membrane-type serine protease 1 | PRSS14 | Prostamin | SNC19 | ST14 | ST14_HUMAN | Serine protease TADG-15 | Suppressor of tumorigenicity 14 protein | Suppressor of tumorigenicity protein 14 | TADG15
Type:
Single-pass type II membrane protein
Mol. Mass.:
94769.23
Organism:
Homo sapiens (Human)
Description:
Q9Y5Y6
Residue:
855
Sequence:
MGSDRARKGGGGPKDFGAGLKYNSRHEKVNGLEEGVEFLPVNNVKKVEKHGPGRWVVLAAVLIGLLLVLLGIGFLVWHLQYRDVRVQKVFNGYMRITNENFVDAYENSNSTEFVSLASKVKDALKLLYSGVPFLGPYHKESAVTAFSEGSVIAYYWSEFSIPQHLVEEAERVMAEERVVMLPPRARSLKSFVVTSVVAFPTDSKTVQRTQDNSCSFGLHARGVELMRFTTPGFPDSPYPAHARCQWALRGDADSVLSLTFRSFDLASCDERGSDLVTVYNTLSPMEPHALVQLCGTYPPSYNLTFHSSQNVLLITLITNTERRHPGFEATFFQLPRMSSCGGRLRKAQGTFNSPYYPGHYPPNIDCTWNIEVPNNQHVKVRFKFFYLLEPGVPAGTCPKDYVEINGEKYCGERSQFVVTSNSNKITVRFHSDQSYTDTGFLAEYLSYDSSDPCPGQFTCRTGRCIRKELRCDGWADCTDHSDELNCSCDAGHQFTCKNKFCKPLFWVCDSVNDCGDNSDEQGCSCPAQTFRCSNGKCLSKSQQCNGKDDCGDGSDEASCPKVNVVTCTKHTYRCLNGLCLSKGNPECDGKEDCSDGSDEKDCDCGLRSFTRQARVVGGTDADEGEWPWQVSLHALGQGHICGASLISPNWLVSAAHCYIDDRGFRYSDPTQWTAFLGLHDQSQRSAPGVQERRLKRIISHPFFNDFTFDYDIALLELEKPAEYSSMVRPICLPDASHVFPAGKAIWVTGWGHTQYGGTGALILQKGEIRVINQTTCENLLPQQITPRMMCVGFLSGGVDSCQGDSGGPLSSVEADGRIFQAGVVSWGDGCAQRNKPGVYTRLPLFRDWIKENTGV
Inhibitor
Name:
BDBM23872
Synonyms:
3-amidinophenylalanine deriv., 16 | 3-{3-[4-(2-carbamimidamidoacetyl)piperazin-1-yl]-3-oxo-2-{[2,4,6-tris(propan-2-yl)benzene]sulfonamido}propyl}benzene-1-carboximidamide
Type:
Small organic molecule
Emp. Form.:
C32H48N8O4S
Mol. Mass.:
640.84
SMILES:
[#6]-[#6](-[#6])-c1cc(-[#6](-[#6])-[#6])c(c(c1)-[#6](-[#6])-[#6])S(=O)(=O)[#7]-[#6](-[#6]-c1cccc(c1)-[#6](-[#7])=[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#7](-[#6]-[#6]-1)-[#6](=O)-[#6]\[#7]=[#6](/[#7])-[#7]
Substrate
Name:
BDBM23868
Synonyms:
(2S)-5-carbamimidamido-2-{2-[(2R)-3-(4-hydroxycyclohexyl)-2-methanesulfonamidopropanamido]acetamido}-N-(4-nitrophenyl)pentanamide | N-Methylsulfonyl-D-hexahydrotyrosyl-glycyl-L-arginine-4-nitroanilide | Pefachrome tPA
Type:
chromogenic substrate
Emp. Form.:
C24H38N8O8S
Mol. Mass.:
598.672
SMILES:
CS(=O)(=O)NC(CC1CCC(O)CC1)C(=O)NCC(=O)NC(CCC\[NH+]=C(/N)[NH-])C(=O)Nc1ccc(cc1)[N+]([O-])=O |(26.51,-19.37,;25.18,-18.6,;24.41,-19.93,;25.95,-17.27,;23.84,-17.83,;22.51,-18.6,;21.18,-17.83,;21.18,-16.29,;22.51,-15.52,;22.51,-13.98,;21.18,-13.21,;21.18,-11.67,;19.84,-13.98,;19.84,-15.52,;22.51,-20.14,;23.84,-20.91,;21.18,-20.91,;21.18,-22.45,;19.84,-23.22,;18.51,-22.45,;19.84,-24.76,;18.51,-25.53,;17.18,-24.76,;17.18,-23.22,;15.84,-22.45,;15.84,-20.91,;14.51,-20.14,;13.17,-20.91,;14.51,-18.6,;18.51,-27.07,;19.84,-27.84,;17.18,-27.84,;17.18,-29.38,;18.51,-30.15,;18.51,-31.69,;17.18,-32.46,;15.84,-31.69,;15.84,-30.15,;17.18,-34,;15.84,-34.77,;18.51,-34.77,)|