Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Bacterial leucyl aminopeptidase
Ligand
BDBM23984
Substrate
BDBM23972
Meas. Tech.
Enzyme Inhibition Assay
IC50
150000±n/a nM
Citation
 Albrecht, S; Defoin, A; Salomon, E; Tarnus, C; Wetterholm, A; Haeggström, JZ Synthesis and structure activity relationships of novel non-peptidic metallo-aminopeptidase inhibitors. Bioorg Med Chem 14:7241-57 (2006) [PubMed] Article
Albrecht, S; Defoin, A; Salomon, E; Tarnus, C; Wetterholm, A; Haeggström, JZ Synthesis and structure activity relationships of novel non-peptidic metallo-aminopeptidase inhibitors. Bioorg Med Chem 14:7241-57 (2006) [PubMed] Article More Info.:
Target
Name:
Bacterial leucyl aminopeptidase
Synonyms:
AMPX_VIBPR | Bacterial leucyl aminopeptidase precursor
Type:
PROTEIN
Mol. Mass.:
54213.72
Organism:
Vibrio proteolyticus
Description:
ChEMBL_1507540
Residue:
504
Sequence:
MKYTKTLLAMVLSATFCQAYAEDKVWISIGADANQTVMKSGAESILPNSVASSGQVWVGQVDVAQLAELSHNMHEEHNRCGGYMVHPSAQSAMAASAMPTTLASFVMPPITQQATVTAWLPQVDASQITGTISSLESFTNRFYTTTSGAQASDWIASEWQALSASLPNASVKQVSHSGYNQKSVVMTITGSEAPDEWIVIGGHLDSTIGSHTNEQSVAPGADDDASGIAAVTEVIRVLSENNFQPKRSIAFMAYAAEEVGLRGSQDLANQYKSEGKNVVSALQLDMTNYKGSAQDVVFITDYTDSNFTQYLTQLMDEYLPSLTYGFDTCGYACSDHASWHNAGYPAAMPFESKFNDYNPRIHTTQDTLANSDPTGSHAKKFTQLGLAYAIEMGSATGDTPTPGNQLEDGVPVTDLSGSRGSNVWYTFELETQKNLQITTSGGYGDLDLYVKFGSKASKQNWDCRPYLSGNNEVCTFNNASPGTYSVMLTGYSNYSGASLKASTF
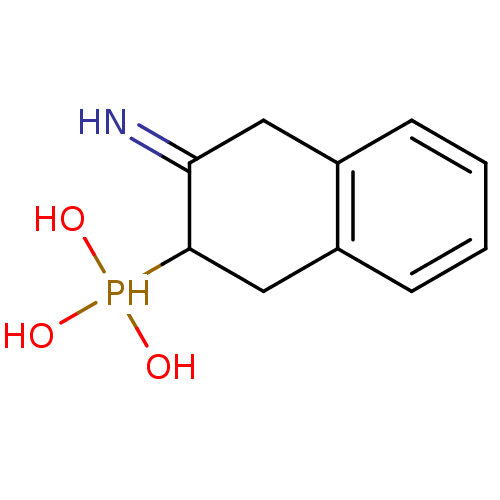
Inhibitor
Name:
BDBM23984
Synonyms:
3-amino-1,2,3,4-tetrahydronaphthalene-2-phosphonic acid hydrochloride | tetrahydronaphthalene-2-phosphonic acid, 3d
Type:
Small organic molecule
Emp. Form.:
C10H14NO3P
Mol. Mass.:
227.1968
SMILES:
OP(O)(O)C1Cc2ccccc2CC1=N