Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Indoleamine 2,3-dioxygenase 1
Ligand
BDBM24819
Substrate
BDBM21974
Meas. Tech.
Enzyme Inhibition Assay
pH
6.5±n/a
Temperature
310.15±n/a K
Ki
47570±n/a nM
Citation
 Gaspari, P; Banerjee, T; Malachowski, WP; Muller, AJ; Prendergast, GC; DuHadaway, J; Bennett, S; Donovan, AM Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J Med Chem 49:684-92 (2006) [PubMed] Article
Gaspari, P; Banerjee, T; Malachowski, WP; Muller, AJ; Prendergast, GC; DuHadaway, J; Bennett, S; Donovan, AM Structure-activity study of brassinin derivatives as indoleamine 2,3-dioxygenase inhibitors. J Med Chem 49:684-92 (2006) [PubMed] Article More Info.:
Target
Name:
Indoleamine 2,3-dioxygenase 1
Synonyms:
I23O1_HUMAN | IDO | IDO-1 | IDO1 | INDO | Indoleamine 2,3-Dioxygenasae (IDO) | Indoleamine 2,3-dioxygenase | Indoleamine-pyrrole 2,3-dioxygenase
Type:
Enzyme
Mol. Mass.:
45330.80
Organism:
Homo sapiens (Human)
Description:
P14902
Residue:
403
Sequence:
MAHAMENSWTISKEYHIDEEVGFALPNPQENLPDFYNDWMFIAKHLPDLIESGQLRERVEKLNMLSIDHLTDHKSQRLARLVLGCITMAYVWGKGHGDVRKVLPRNIAVPYCQLSKKLELPPILVYADCVLANWKKKDPNKPLTYENMDVLFSFRDGDCSKGFFLVSLLVEIAAASAIKVIPTVFKAMQMQERDTLLKALLEIASCLEKALQVFHQIHDHVNPKAFFSVLRIYLSGWKGNPQLSDGLVYEGFWEDPKEFAGGSAGQSSVFQCFDVLLGIQQTAGGGHAAQFLQDMRRYMPPAHRNFLCSLESNPSVREFVLSKGDAGLREAYDACVKALVSLRSYHLQIVTKYILIPASQQPKENKTSEDPSKLEAKGTGGTDLMNFLKTVRSTTEKSLLKEG
Inhibitor
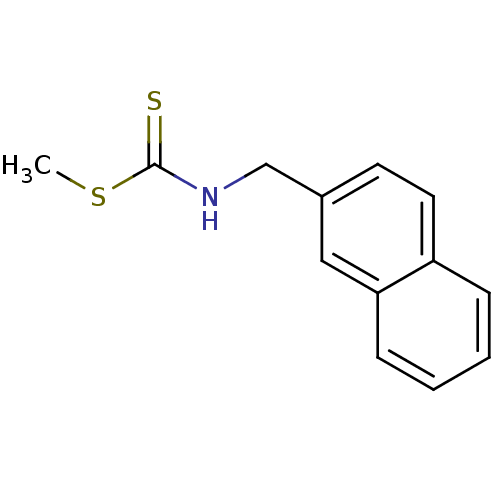
Name:
BDBM24819
Synonyms:
(methylsulfanyl)-N-(naphthalen-2-ylmethyl)carbothioamide | Brassinin derivative, 7
Type:
Small organic molecule
Emp. Form.:
C13H13NS2
Mol. Mass.:
247.379
SMILES:
CSC(=S)NCc1ccc2ccccc2c1