Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neutral cholesterol ester hydrolase 1
Ligand
BDBM26736
Substrate
BDBM26737
Meas. Tech.
Serine Hydrolase Activity Assay
pH
7.4±n/a
Temperature
295.15±n/a K
IC50
8.2±n/a nM
Citation
 Alexander, JP; Cravatt, BF The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc 128:9699-704 (2006) [PubMed] Article
Alexander, JP; Cravatt, BF The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. J Am Chem Soc 128:9699-704 (2006) [PubMed] Article More Info.:
Target
Name:
Neutral cholesterol ester hydrolase 1
Synonyms:
Aadacl1 | Arylacetamide deacetylase-like 1 | CPO-BP | Chlorpyrifos oxon-binding protein | Kiaa1363 | NCEH | NCEH1_MOUSE | Nceh1 | Neutral cholesterol ester hydrolase
Type:
Single-pass type II membrane protein; hydrolase
Mol. Mass.:
45742.11
Organism:
Mus musculus (mouse)
Description:
Assays were using membranes of recombinant KIAA1363 transiently transfected in COS-7 cells.
Residue:
408
Sequence:
MRSSCVLLAALLALAAYYVYIPLPSAVSDPWKLMLLDATFRGAQQVSNLIHSLGLNHHLIALNFIITSFGKQSARSSPKVKVTDTDFDGVEVRVFEGSPKPEEPLRRSVIYIHGGGWALASAKISYYDQLCTTMAEELNAVIVSIEYRLVPQVYFPEQIHDVIRATKYFLQPEVLDKYKVDPGRVGISGDSAGGNLAAALGQQFTYVASLKNKLKLQALVYPVLQALDFNTPSYQQSMNTPILPRHVMVRYWLDYFKGNYDFVEAMIVNNHTSLDVERAAALRARLDWTSLLPSSIKKNYKPIMQTTGNARIVQEIPQLLDAAASPLIAEQEVLEALPKTYILTCEHDVLRDDGIMYAKRLESAGVNVTLDHFEDGFHGCMIFTSWPTNFSVGIRTRNSYIKWLDQNL
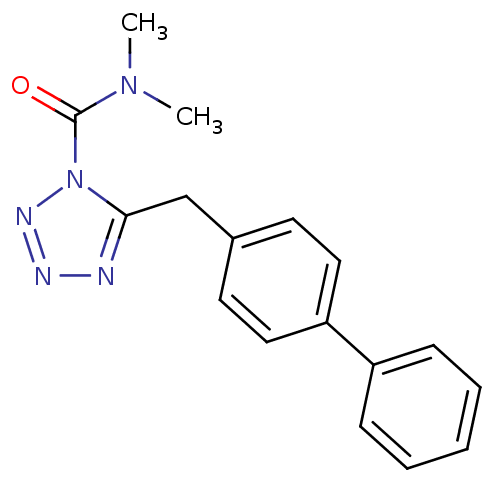
Inhibitor
Name:
BDBM26736
Synonyms:
CHEMBL509860 | LY2183240 | N,N-dimethyl-5-[(4-phenylphenyl)methyl]-1H-1,2,3,4-tetrazole-1-carboxamide
Type:
Small organic molecule
Emp. Form.:
C17H17N5O
Mol. Mass.:
307.3498
SMILES:
CN(C)C(=O)n1nnnc1Cc1ccc(cc1)-c1ccccc1
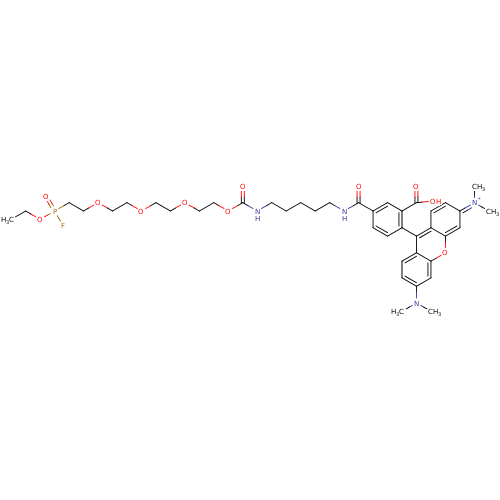
Substrate
Name:
BDBM26737
Synonyms:
9-{2-carboxy-4-[(5-{14-[ethoxy(fluoro)phosphoryl]-2-oxo-3,6,9,12-tetraoxa-1-azatetradecan-1-yl}pentyl)carbamoyl]phenyl}-6-(dimethylamino)-N,N-dimethyl-3H-xanthen-3-iminium | FP-rhodamine | rhodamine-tagged fluorophosphonate
Type:
n/a
Emp. Form.:
C41H55FN4O11P
Mol. Mass.:
829.8672
SMILES:
CCOP(F)(=O)CCOCCOCCOCCOC(=O)NCCCCCNC(=O)c1ccc(c(c1)C(O)=O)-c1c2ccc(cc2oc2cc(ccc12)=[N+](C)C)N(C)C |(28.8,3.82,;28.03,5.15,;26.49,5.15,;25.72,6.49,;27.2,6.89,;24.95,7.82,;24.38,5.72,;23.05,6.49,;21.72,5.72,;20.38,6.49,;19.05,5.72,;17.71,6.49,;16.38,5.72,;15.05,6.49,;13.71,5.72,;12.38,6.49,;11.05,5.72,;9.71,6.49,;8.38,5.72,;8.38,4.18,;7.04,6.49,;5.71,5.72,;4.38,6.49,;3.04,5.72,;1.71,6.49,;.38,5.72,;-.96,6.49,;-2.29,5.72,;-3.62,6.49,;-2.29,4.18,;-.96,3.41,;-.96,1.87,;-2.29,1.1,;-3.62,1.87,;-3.62,3.41,;-5.11,1.47,;-6.2,2.56,;-5.51,-.02,;-2.29,-.44,;-.96,-1.21,;.38,-.44,;1.71,-1.21,;1.71,-2.75,;.38,-3.52,;-.96,-2.75,;-2.29,-3.52,;-3.62,-2.75,;-4.96,-3.52,;-6.29,-2.75,;-6.29,-1.21,;-4.96,-.44,;-3.62,-1.21,;-7.63,-3.52,;-7.63,-5.06,;-8.96,-2.75,;3.04,-3.52,;4.38,-2.75,;3.04,-5.06,)|