Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Fibroblast growth factor receptor 2
Ligand
BDBM287528
Substrate
n/a
Meas. Tech.
Inhibition of FGFR2-Dependent Cell Growth
IC50
4.20±n/a nM
Citation
 Verner, E; Brameld, KA Quinolone derivatives as fibroblast growth factor receptor inhibitors US Patent US10294223 Publication Date 5/21/2019
Verner, E; Brameld, KA Quinolone derivatives as fibroblast growth factor receptor inhibitors US Patent US10294223 Publication Date 5/21/2019 More Info.:
Target
Name:
Fibroblast growth factor receptor 2
Synonyms:
BEK | CD_antigen=CD332 | FGFR-2 | FGFR-2 Tyrosine Kinase | FGFR2 | FGFR2_HUMAN | Fibroblast growth factor receptor 2 (FGFR2) | Fibroblast growth factor receptor 2 precursor | KGFR | KSAM | Keratinocyte growth factor receptor | Keratinocyte growth factor receptor 2 | VEGF-receptor 2 and Fibroblast growth factor receptor 2
Type:
Enzyme
Mol. Mass.:
92015.45
Organism:
Homo sapiens (Human)
Description:
P21802
Residue:
821
Sequence:
MVSWGRFICLVVVTMATLSLARPSFSLVEDTTLEPEEPPTKYQISQPEVYVAAPGESLEVRCLLKDAAVISWTKDGVHLGPNNRTVLIGEYLQIKGATPRDSGLYACTASRTVDSETWYFMVNVTDAISSGDDEDDTDGAEDFVSENSNNKRAPYWTNTEKMEKRLHAVPAANTVKFRCPAGGNPMPTMRWLKNGKEFKQEHRIGGYKVRNQHWSLIMESVVPSDKGNYTCVVENEYGSINHTYHLDVVERSPHRPILQAGLPANASTVVGGDVEFVCKVYSDAQPHIQWIKHVEKNGSKYGPDGLPYLKVLKAAGVNTTDKEIEVLYIRNVTFEDAGEYTCLAGNSIGISFHSAWLTVLPAPGREKEITASPDYLEIAIYCIGVFLIACMVVTVILCRMKNTTKKPDFSSQPAVHKLTKRIPLRRQVTVSAESSSSMNSNTPLVRITTRLSSTADTPMLAGVSEYELPEDPKWEFPRDKLTLGKPLGEGCFGQVVMAEAVGIDKDKPKEAVTVAVKMLKDDATEKDLSDLVSEMEMMKMIGKHKNIINLLGACTQDGPLYVIVEYASKGNLREYLRARRPPGMEYSYDINRVPEEQMTFKDLVSCTYQLARGMEYLASQKCIHRDLAARNVLVTENNVMKIADFGLARDINNIDYYKKTTNGRLPVKWMAPEALFDRVYTHQSDVWSFGVLMWEIFTLGGSPYPGIPVEELFKLLKEGHRMDKPANCTNELYMMMRDCWHAVPSQRPTFKQLVEDLDRILTLTTNEEYLDLSQPLEQYSPSYPDTRSSCSSGDDSVFSPDPMPYEPCLPQYPHINGSVKT
Inhibitor
Name:
BDBM287528
Synonyms:
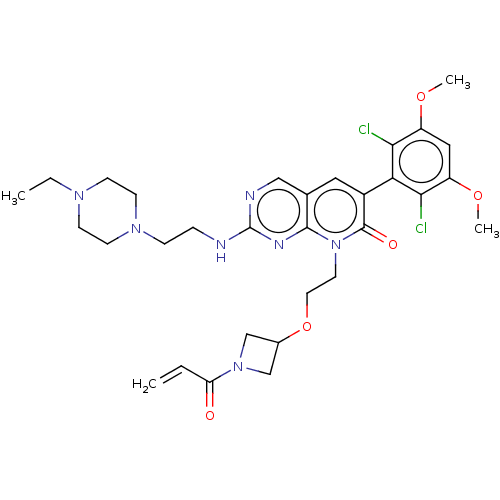
8-(2-((1-acryloylazetidin-3-yl)oxy)ethyl)-6-(2,6-dichloro-3,5-dimethoxyphenyl)-2-((2-(4-ethylpiperazin-1-yl)ethyl)amino)pyrido[2,3-d]pyrimidin-7(8H)-one | US10294223, Cpd No. 94 | US11078199, Cpd 94 | US9567334, Example 94 | US9815834, Compound 94
Type:
Small organic molecule
Emp. Form.:
C31H39Cl2N7O5
Mol. Mass.:
660.591
SMILES:
CCN1CCN(CCNc2ncc3cc(-c4c(Cl)c(OC)cc(OC)c4Cl)c(=O)n(CCOC4CN(C4)C(=O)C=C)c3n2)CC1 |(-5.61,-8.47,;-6.94,-7.7,;-6.94,-6.16,;-8.28,-5.39,;-8.28,-3.85,;-6.94,-3.08,;-6.94,-1.54,;-5.61,-.77,;-5.61,.77,;-4.28,1.54,;-4.28,3.08,;-2.94,3.85,;-1.61,3.08,;-.28,3.85,;1.06,3.08,;2.39,3.85,;2.39,5.39,;1.06,6.16,;3.72,6.16,;3.72,7.7,;5.06,8.47,;5.06,5.39,;5.06,3.85,;6.39,3.08,;7.73,3.85,;3.72,3.08,;3.72,1.54,;1.06,1.54,;2.39,.77,;-.28,.77,;-.28,-.77,;1.06,-1.54,;1.06,-3.08,;2.39,-3.85,;2.79,-5.34,;4.28,-4.94,;3.88,-3.45,;5.61,-5.71,;5.61,-7.25,;6.94,-4.94,;8.28,-5.71,;-1.61,1.54,;-2.94,.77,;-5.61,-3.85,;-5.61,-5.39,)|