Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Poly [ADP-ribose] polymerase 1
Ligand
BDBM27525
Substrate
BDBM27089
Meas. Tech.
PARP ELISA Assay
pH
8±n/a
Temperature
295.15±n/a K
IC50
1±n/a nM
Citation
 Jagtap, PG; Baloglu, E; Southan, GJ; Mabley, JG; Li, H; Zhou, J; van Duzer, J; Salzman, AL; Szabó, C Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J Med Chem 48:5100-3 (2005) [PubMed] Article
Jagtap, PG; Baloglu, E; Southan, GJ; Mabley, JG; Li, H; Zhou, J; van Duzer, J; Salzman, AL; Szabó, C Discovery of potent poly(ADP-ribose) polymerase-1 inhibitors from the modification of indeno[1,2-c]isoquinolinone. J Med Chem 48:5100-3 (2005) [PubMed] Article More Info.:
Target
Name:
Poly [ADP-ribose] polymerase 1
Synonyms:
(ARTD1 or PARP1) | 2.4.2.- | 2.4.2.30 | ADP-ribosyltransferase diphtheria toxin-like 1 | ADPRT | ADPRT 1 | ARTD1 | DNA ADP-ribosyltransferase PARP1 | Human diphtheria toxin-like ADP-ribosyltransferase (ARTD1 or PARP1) | NAD(+) ADP-ribosyltransferase 1 | NT-PARP-1 | PARP-1 | PARP1 | PARP1_HUMAN | PPOL | Poly [ADP-ribose] polymerase (PARP) | Poly [ADP-ribose] polymerase 1 (PARP) | Poly [ADP-ribose] polymerase 1 (PARP-1) | Poly [ADP-ribose] polymerase 1 (PARP1) | Poly [ADP-ribose] polymerase 1, 24-kDa form | Poly [ADP-ribose] polymerase 1, 28-kDa form | Poly [ADP-ribose] polymerase 1, 89-kDa form | Poly [ADP-ribose] polymerase 1, processed C-terminus | Poly [ADP-ribose] polymerase 1, processed N-terminus | Poly [ADP-ribose] polymerase-1 | Poly(ADP-ribose) polymerase 1 (PARP1) | Poly(ADP-ribose) polymerase-1 (ARTD1/PARP1) | Poly[ADP-ribose] synthase 1 | Protein poly-ADP-ribosyltransferase PARP1 | Synonyms=ADPRT
Type:
n/a
Mol. Mass.:
113114.22
Organism:
Homo sapiens (Human)
Description:
P09874
Residue:
1014
Sequence:
MAESSDKLYRVEYAKSGRASCKKCSESIPKDSLRMAIMVQSPMFDGKVPHWYHFSCFWKVGHSIRHPDVEVDGFSELRWDDQQKVKKTAEAGGVTGKGQDGIGSKAEKTLGDFAAEYAKSNRSTCKGCMEKIEKGQVRLSKKMVDPEKPQLGMIDRWYHPGCFVKNREELGFRPEYSASQLKGFSLLATEDKEALKKQLPGVKSEGKRKGDEVDGVDEVAKKKSKKEKDKDSKLEKALKAQNDLIWNIKDELKKVCSTNDLKELLIFNKQQVPSGESAILDRVADGMVFGALLPCEECSGQLVFKSDAYYCTGDVTAWTKCMVKTQTPNRKEWVTPKEFREISYLKKLKVKKQDRIFPPETSASVAATPPPSTASAPAAVNSSASADKPLSNMKILTLGKLSRNKDEVKAMIEKLGGKLTGTANKASLCISTKKEVEKMNKKMEEVKEANIRVVSEDFLQDVSASTKSLQELFLAHILSPWGAEVKAEPVEVVAPRGKSGAALSKKSKGQVKEEGINKSEKRMKLTLKGGAAVDPDSGLEHSAHVLEKGGKVFSATLGLVDIVKGTNSYYKLQLLEDDKENRYWIFRSWGRVGTVIGSNKLEQMPSKEDAIEHFMKLYEEKTGNAWHSKNFTKYPKKFYPLEIDYGQDEEAVKKLTVNPGTKSKLPKPVQDLIKMIFDVESMKKAMVEYEIDLQKMPLGKLSKRQIQAAYSILSEVQQAVSQGSSDSQILDLSNRFYTLIPHDFGMKKPPLLNNADSVQAKVEMLDNLLDIEVAYSLLRGGSDDSSKDPIDVNYEKLKTDIKVVDRDSEEAEIIRKYVKNTHATTHNAYDLEVIDIFKIEREGECQRYKPFKQLHNRRLLWHGSRTTNFAGILSQGLRIAPPEAPVTGYMFGKGIYFADMVSKSANYCHTSQGDPIGLILLGEVALGNMYELKHASHISKLPKGKHSVKGLGKTTPDPSANISLDGVDVPLGTGISSGVNDTSLLYNEYIVYDIAQVNLKYLLKLKFNFKTSLW
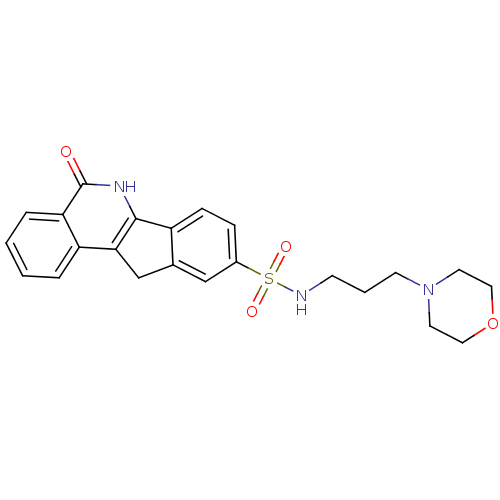
Inhibitor
Name:
BDBM27525
Synonyms:
N-[3-(morpholin-4-yl)propyl]-8-oxo-9-azatetracyclo[8.7.0.0^{2,7}.0^{11,16}]heptadeca-1(10),2(7),3,5,11(16),12,14-heptaene-14-sulfonamide | indeno[1,2-c]isoquinolinone, 11a
Type:
Small organic molecule
Emp. Form.:
C23H25N3O4S
Mol. Mass.:
439.527
SMILES:
O=c1[nH]c-2c(Cc3cc(ccc-23)S(=O)(=O)NCCCN2CCOCC2)c2ccccc12
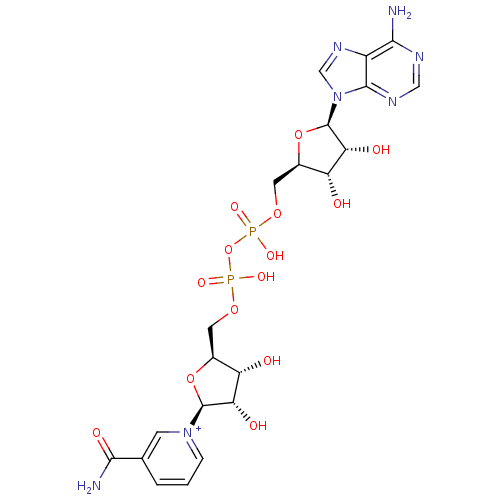
Substrate
Name:
BDBM27089
Synonyms:
[3H] Nicotinamide adenine dinucleotide | [3H]NAD+ | biotinylated NAD+
Type:
radiolabeled substrate
Emp. Form.:
C21H28N7O14P2
Mol. Mass.:
664.4325
SMILES:
NC(=O)c1ccc[n+](c1)[C@H]1O[C@@H](COP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)n2cnc3c(N)ncnc23)[C@H](O)[C@@H]1O