Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
D(1A) dopamine receptor
Ligand
BDBM50241107
Substrate
n/a
Ki
2037±n/a nM
Comments
PDSP_726
Citation
 Simonneaux, V; Murrin, LC; Ebadi, M Characterization of D1 dopamine receptors in the bovine pineal gland with [3H]SCH 23390. J Pharmacol Exp Ther 253:214-20 (1990) [PubMed]
Simonneaux, V; Murrin, LC; Ebadi, M Characterization of D1 dopamine receptors in the bovine pineal gland with [3H]SCH 23390. J Pharmacol Exp Ther 253:214-20 (1990) [PubMed] More Info.:
Target
Name:
D(1A) dopamine receptor
Synonyms:
D(1A) dopamine receptor | D1AR | DOPAMINE D1 | DRD1 | DRD1_BOVIN | Dopamine receptor
Type:
Enzyme Catalytic Domain
Mol. Mass.:
49330.65
Organism:
BOVINE
Description:
DOPAMINE D1 DRD1 BOVINE::Q95136
Residue:
446
Sequence:
MRTLNTSTMEGTGLVAERDFSFRILTACFLSLLILSTLLGNTLVCAAVIRFRHLRSKVTNFFVISLAVSDLLVAVLVMPWKAVAEIAGFWPFGSFCNIWVAFDIMCSTASILNLCVISVDRYWAISSPFRYERKMTPKAAFILISVAWTLSVLISFIPVQLSWHKAKPTGPSEGNATSLGKTINNCDSSLSRTYAISSSLISFYIPVAIMIVTYTRIYRIAQKQIRRISALERAAVHAKNCQTTTGNGNPMECSQPESSFKMSFKRETKVLKTLSVIMGVFVCCWLPFFILNCMVPFCGSGETKPFCIDSITFDVFVWFGWANSSLNPIIYAFNADFRKAFSTLLGCYRLCPTTNNAIETVSINNNGAVVFSSHHEPRGSISKDCNVVYLIPHAVGSSEGLKKEEAVGIAKPLEKLSPALSVILDYDTDVSLEKIQPITQNGQHPT
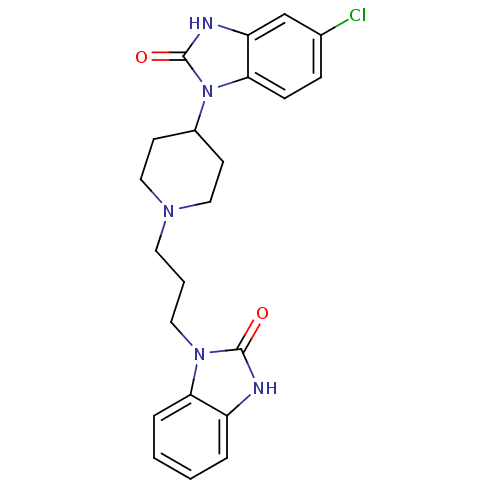
Inhibitor
Name:
BDBM50241107
Synonyms:
1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazol-1-yl)piperidin-1-yl)propyl)-1H-benzo[d]imidazol-2(3H)-one | 5-Chloro-1-{1-[3-(2-hydroxy-2,3-dihydro-benzoimidazol-1-yl)-propyl]-piperidin-4-yl}-2,3-dihydro-1H-benzoimidazol-2-ol | 5-Chloro-1-{1-[3-(2-oxo-2,3-dihydro-benzoimidazol-1-yl)-propyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one(domperidone) | 5-chloro-1-(1-(3-(2-oxo-2,3-dihydrobenzo[d]imidazol-1-yl)propyl)piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one | 5-chloro-1-{1-[3-(2-oxo-2,3-dihydro-benzoimidazol-1-yl)-propyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one | 6-Chloro-1-{1-[3-(2-oxo-2,3-dihydro-benzoimidazol-1-yl)-propyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one(Domperidone) | CHEMBL219916 | DOMPERIDONE | DOMPERIDONE5-Chloro-1-{1-[3-(2-oxo-2,3-dihydro-benzoimidazol-1-yl)-propyl]-piperidin-4-yl}-1,3-dihydro-benzoimidazol-2-one | US11147820, Compound Domperidone | US9132134, Domperidone
Type:
Small organic molecule
Emp. Form.:
C22H24ClN5O2
Mol. Mass.:
425.911
SMILES:
Clc1ccc2n(C3CCN(CCCn4c5ccccc5[nH]c4=O)CC3)c(=O)[nH]c2c1