Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Gag-Pol polyprotein
Ligand
BDBM50450015
Substrate
n/a
Meas. Tech.
ChEMBL_1741650 (CHEMBL4157400)
Kd
6.5±n/a nM
Citation
 Regueiro-Ren, A; Swidorski, JJ; Liu, Z; Chen, Y; Sin, N; Sit, SY; Chen, J; Venables, BL; Zhu, J; Nowicka-Sans, B; Protack, T; Lin, Z; Terry, B; Samanta, H; Zhang, S; Li, Z; Easter, J; Beno, BR; Arora, V; Huang, XS; Rahematpura, S; Parker, DD; Haskell, R; Santone, KS; Cockett, MI; Krystal, M; Meanwell, NA; Jenkins, S; Hanumegowda, U; Dicker, IB Design, Synthesis, and SAR of C-3 Benzoic Acid, C-17 Triterpenoid Derivatives. Identification of the HIV-1 Maturation Inhibitor 4-((1 R,3a S,5a R,5b R,7a R,11a S,11b R,13a R,13b R)-3a-((2-(1,1-Dioxidothiomorpholino)ethyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,1 J Med Chem 61:7289-7313 (2018) [PubMed] Article
Regueiro-Ren, A; Swidorski, JJ; Liu, Z; Chen, Y; Sin, N; Sit, SY; Chen, J; Venables, BL; Zhu, J; Nowicka-Sans, B; Protack, T; Lin, Z; Terry, B; Samanta, H; Zhang, S; Li, Z; Easter, J; Beno, BR; Arora, V; Huang, XS; Rahematpura, S; Parker, DD; Haskell, R; Santone, KS; Cockett, MI; Krystal, M; Meanwell, NA; Jenkins, S; Hanumegowda, U; Dicker, IB Design, Synthesis, and SAR of C-3 Benzoic Acid, C-17 Triterpenoid Derivatives. Identification of the HIV-1 Maturation Inhibitor 4-((1 R,3a S,5a R,5b R,7a R,11a S,11b R,13a R,13b R)-3a-((2-(1,1-Dioxidothiomorpholino)ethyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,1 J Med Chem 61:7289-7313 (2018) [PubMed] Article More Info.:
Target
Name:
Gag-Pol polyprotein
Synonyms:
HIV-1 reverse transcriptase (HIV-1 RT) | Integrase | POL_HV1H2 | Pr160Gag-Pol | Protease | Reverse transcriptase (HIV-1 RT) | Reverse transcriptase (RT) | Ribonuclease H (RNase H) | gag-pol | p51 RT | p66 RT
Type:
Protein
Mol. Mass.:
162074.74
Organism:
Human immunodeficiency virus type 1 group M subtype B (isolate HXB2)
Description:
P04585
Residue:
1435
Sequence:
MGARASVLSGGELDRWEKIRLRPGGKKKYKLKHIVWASRELERFAVNPGLLETSEGCRQILGQLQPSLQTGSEELRSLYNTVATLYCVHQRIEIKDTKEALDKIEEEQNKSKKKAQQAAADTGHSNQVSQNYPIVQNIQGQMVHQAISPRTLNAWVKVVEEKAFSPEVIPMFSALSEGATPQDLNTMLNTVGGHQAAMQMLKETINEEAAEWDRVHPVHAGPIAPGQMREPRGSDIAGTTSTLQEQIGWMTNNPPIPVGEIYKRWIILGLNKIVRMYSPTSILDIRQGPKEPFRDYVDRFYKTLRAEQASQEVKNWMTETLLVQNANPDCKTILKALGPAATLEEMMTACQGVGGPGHKARVLAEAMSQVTNSATIMMQRGNFRNQRKIVKCFNCGKEGHTARNCRAPRKKGCWKCGKEGHQMKDCTERQANFLREDLAFLQGKAREFSSEQTRANSPTRRELQVWGRDNNSPSEAGADRQGTVSFNFPQVTLWQRPLVTIKIGGQLKEALLDTGADDTVLEEMSLPGRWKPKMIGGIGGFIKVRQYDQILIEICGHKAIGTVLVGPTPVNIIGRNLLTQIGCTLNFPISPIETVPVKLKPGMDGPKVKQWPLTEEKIKALVEICTEMEKEGKISKIGPENPYNTPVFAIKKKDSTKWRKLVDFRELNKRTQDFWEVQLGIPHPAGLKKKKSVTVLDVGDAYFSVPLDEDFRKYTAFTIPSINNETPGIRYQYNVLPQGWKGSPAIFQSSMTKILEPFRKQNPDIVIYQYMDDLYVGSDLEIGQHRTKIEELRQHLLRWGLTTPDKKHQKEPPFLWMGYELHPDKWTVQPIVLPEKDSWTVNDIQKLVGKLNWASQIYPGIKVRQLCKLLRGTKALTEVIPLTEEAELELAENREILKEPVHGVYYDPSKDLIAEIQKQGQGQWTYQIYQEPFKNLKTGKYARMRGAHTNDVKQLTEAVQKITTESIVIWGKTPKFKLPIQKETWETWWTEYWQATWIPEWEFVNTPPLVKLWYQLEKEPIVGAETFYVDGAANRETKLGKAGYVTNRGRQKVVTLTDTTNQKTELQAIYLALQDSGLEVNIVTDSQYALGIIQAQPDQSESELVNQIIEQLIKKEKVYLAWVPAHKGIGGNEQVDKLVSAGIRKVLFLDGIDKAQDEHEKYHSNWRAMASDFNLPPVVAKEIVASCDKCQLKGEAMHGQVDCSPGIWQLDCTHLEGKVILVAVHVASGYIEAEVIPAETGQETAYFLLKLAGRWPVKTIHTDNGSNFTGATVRAACWWAGIKQEFGIPYNPQSQGVVESMNKELKKIIGQVRDQAEHLKTAVQMAVFIHNFKRKGGIGGYSAGERIVDIIATDIQTKELQKQITKIQNFRVYYRDSRNPLWKGPAKLLWKGEGAVVIQDNSDIKVVPRRKAKIIRDYGKQMAGDDCVASRQDED
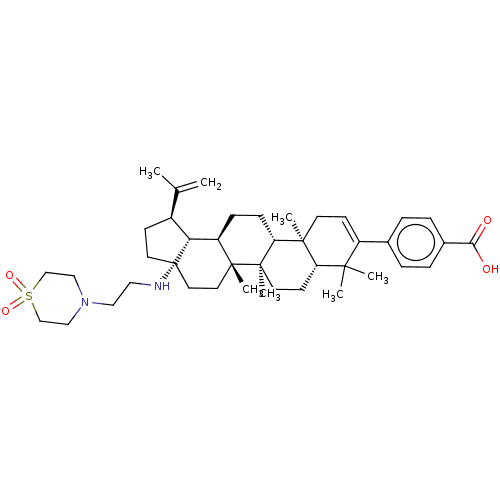
Inhibitor
Name:
BDBM50450015
Synonyms:
Bms-955176 | GSK-3532795
Type:
Small organic molecule
Emp. Form.:
C42H62N2O4S
Mol. Mass.:
691.018
SMILES:
[H][C@]12[C@@H](CC[C@@]1(CC[C@]1(C)[C@]2([H])CC[C@]2([H])[C@@]3(C)CC=C(c4ccc(cc4)C(O)=O)C(C)(C)[C@]3([H])CC[C@@]12C)NCCN1CCS(=O)(=O)CC1)C(C)=C |r,t:21|