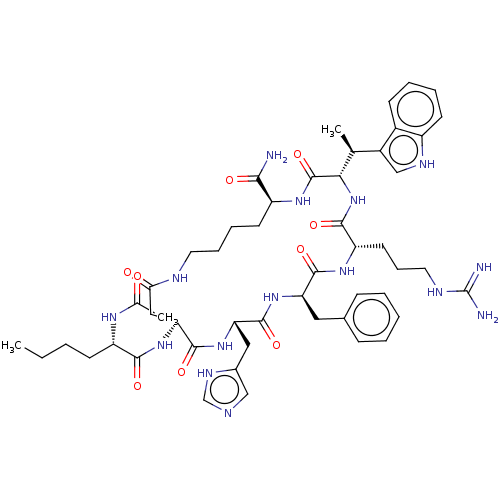
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Melanocyte-stimulating hormone receptor
Ligand
BDBM50029748
Substrate
n/a
Meas. Tech.
ChEMBL_105822 (CHEMBL716482)
EC50
0.4±n/a nM
Citation
 Haskell-Luevano, C; Boteju, LW; Miwa, H; Dickinson, C; Gantz, I; Yamada, T; Hadley, ME; Hruby, VJ Topographical modification of melanotropin peptide analogues with beta-methyltryptophan isomers at position 9 leads to differential potencies and prolonged biological activities. J Med Chem 38:4720-9 (1995) [PubMed] Article
Haskell-Luevano, C; Boteju, LW; Miwa, H; Dickinson, C; Gantz, I; Yamada, T; Hadley, ME; Hruby, VJ Topographical modification of melanotropin peptide analogues with beta-methyltryptophan isomers at position 9 leads to differential potencies and prolonged biological activities. J Med Chem 38:4720-9 (1995) [PubMed] Article More Info.:
Target
Name:
Melanocyte-stimulating hormone receptor
Synonyms:
MC1-R | MC1R | MSH-R | MSHR | MSHR_HUMAN | Melanocortin MC1 | Melanocortin receptor (M1 and M4) | Melanocortin receptor 1 (MC-1) | Melanocortin receptor 1 (MC1-R) | Melanocortin receptor 1 (MC1R)
Type:
Enzyme
Mol. Mass.:
34717.23
Organism:
Homo sapiens (Human)
Description:
Q01726
Residue:
317
Sequence:
MAVQGSQRRLLGSLNSTPTAIPQLGLAANQTGARCLEVSISDGLFLSLGLVSLVENALVVATIAKNRNLHSPMYCFICCLALSDLLVSGSNVLETAVILLLEAGALVARAAVLQQLDNVIDVITCSSMLSSLCFLGAIAVDRYISIFYALRYHSIVTLPRARRAVAAIWVASVVFSTLFIAYYDHVAVLLCLVVFFLAMLVLMAVLYVHMLARACQHAQGIARLHKRQRPVHQGFGLKGAVTLTILLGIFFLCWGPFFLHLTLIVLCPEHPTCGCIFKNFNLFLALIICNAIIDPLIYAFHSQELRRTLKEVLTCSW
Inhibitor
Name:
BDBM50029748
Synonyms:
(2R,3R)-15-(2-Acetylamino-hexanoylamino)-9-benzyl-6-(3-guanidino-propyl)-12-(3H-imidazol-4-ylmethyl)-3-(1H-indol-3-ylmethyl)-4-methyl-2,5,8,11,14,17-hexaoxo-1,4,7,10,13,18hexaaza-cyclotricosane-23-carboxylic acid amide | CHEMBL2364555
Type:
Small organic molecule
Emp. Form.:
C51H71N15O9
Mol. Mass.:
1038.2045
SMILES:
[H][C@]1(NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](CC(=O)NCCCC[C@H](NC1=O)C(N)=O)NC(=O)[C@H](CCCC)NC(C)=O)[C@@H](C)c1c[nH]c2ccccc12 |wU:27.28,56.63,65.68,5.5,wD:37.55,46.52,16.16,1.0,(19.89,-7.11,;18.68,-8.53,;18.45,-7.02,;17.78,-5.61,;19.09,-4.78,;16.79,-4.45,;17.81,-3.3,;19.32,-3.62,;20.34,-2.47,;21.85,-2.76,;22.87,-1.63,;24.37,-1.93,;22.39,-.17,;15.51,-3.6,;14.03,-3.12,;13.68,-4.91,;12.5,-3.04,;12.37,-1.5,;13.62,-.62,;13.49,.92,;14.74,1.81,;16.15,1.17,;16.27,-.36,;15.03,-1.26,;11.02,-3.39,;9.65,-4.13,;10.31,-5.51,;8.56,-5.2,;7.34,-4.26,;7.57,-2.73,;8.91,-2,;8.62,-.49,;7.12,-.27,;6.45,-1.66,;7.79,-6.54,;7.41,-8.01,;5.87,-7.85,;7.44,-9.54,;7.89,-11.05,;8.72,-12.34,;7.57,-13.33,;9.87,-13.36,;11.25,-14.03,;12.78,-14.32,;14.29,-14.18,;15.73,-13.64,;16.98,-12.71,;17.94,-11.49,;18.52,-10.06,;19.99,-10.45,;18.07,-13.81,;17.65,-15.3,;19.54,-13.43,;5.93,-9.81,;5.36,-11.25,;6.35,-12.45,;3.85,-11.49,;2.89,-10.29,;3.44,-8.87,;2.44,-7.66,;2.99,-6.22,;3.31,-12.93,;1.8,-13.27,;1.32,-14.71,;.78,-12.11,;20.21,-8.52,;20.6,-7.02,;21.56,-9.26,;23,-8.75,;23.93,-9.99,;23.03,-11.25,;23.41,-12.75,;22.26,-13.81,;20.79,-13.36,;20.47,-11.86,;21.59,-10.8,)|