Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mitogen-activated protein kinase 9
Ligand
BDBM13336
Substrate
n/a
Meas. Tech.
ChEMBL_216819 (CHEMBL816398)
IC50
290±n/a nM
Citation
 Liverton, NJ; Butcher, JW; Claiborne, CF; Claremon, DA; Libby, BE; Nguyen, KT; Pitzenberger, SM; Selnick, HG; Smith, GR; Tebben, A; Vacca, JP; Varga, SL; Agarwal, L; Dancheck, K; Forsyth, AJ; Fletcher, DS; Frantz, B; Hanlon, WA; Harper, CF; Hofsess, SJ; Kostura, M; Lin, J; Luell, S; O'Neill, EA; O'Keefe, SJ Design and synthesis of potent, selective, and orally bioavailable tetrasubstituted imidazole inhibitors of p38 mitogen-activated protein kinase. J Med Chem 42:2180-90 (1999) [PubMed] Article
Liverton, NJ; Butcher, JW; Claiborne, CF; Claremon, DA; Libby, BE; Nguyen, KT; Pitzenberger, SM; Selnick, HG; Smith, GR; Tebben, A; Vacca, JP; Varga, SL; Agarwal, L; Dancheck, K; Forsyth, AJ; Fletcher, DS; Frantz, B; Hanlon, WA; Harper, CF; Hofsess, SJ; Kostura, M; Lin, J; Luell, S; O'Neill, EA; O'Keefe, SJ Design and synthesis of potent, selective, and orally bioavailable tetrasubstituted imidazole inhibitors of p38 mitogen-activated protein kinase. J Med Chem 42:2180-90 (1999) [PubMed] Article More Info.:
Target
Name:
Mitogen-activated protein kinase 9
Synonyms:
JNK-55 | JNK2 | JNK2/JNK3 | MAPK9 | MK09_HUMAN | Mitogen-Activated Protein Kinase 9 (JNK2) | Mitogen-activated protein kinase 8/9 | PRKM9 | SAPK1A | Stress-activated protein kinase JNK2 | c-Jun N-terminal kinase 2 | c-Jun N-terminal kinase 2 (JNK2)
Type:
Enzyme
Mol. Mass.:
48131.49
Organism:
Homo sapiens (Human)
Description:
JNK-2 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology).
Residue:
424
Sequence:
MSDSKCDSQFYSVQVADSTFTVLKRYQQLKPIGSGAQGIVCAAFDTVLGINVAVKKLSRPFQNQTHAKRAYRELVLLKCVNHKNIISLLNVFTPQKTLEEFQDVYLVMELMDANLCQVIHMELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTACTNFMMTPYVVTRYYRAPEVILGMGYKENVDIWSVGCIMGELVKGCVIFQGTDHIDQWNKVIEQLGTPSAEFMKKLQPTVRNYVENRPKYPGIKFEELFPDWIFPSESERDKIKTSQARDLLSKMLVIDPDKRISVDEALRHPYITVWYDPAEAEAPPPQIYDAQLEEREHAIEEWKELIYKEVMDWEERSKNGVVKDQPSDAAVSSNATPSQSSSINDISSMSTEQTLASDTDSSLDASTGPLEGCR
Inhibitor
Name:
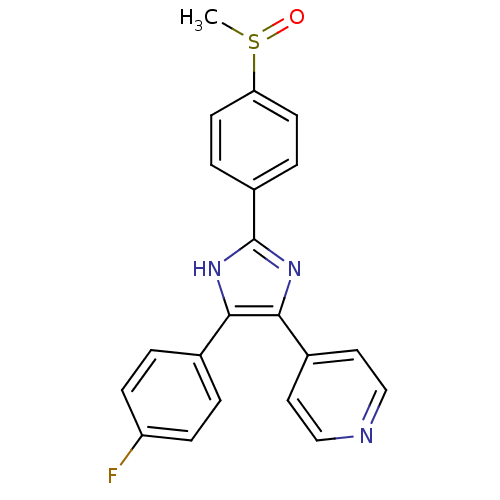
BDBM13336
Synonyms:
4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine | 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine | 4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine | CHEMBL10 | SB-203580 | SB203580 | cid_176155
Type:
Small organic molecule
Emp. Form.:
C21H16FN3OS
Mol. Mass.:
377.435
SMILES:
CS(=O)c1ccc(cc1)-c1nc(c([nH]1)-c1ccc(F)cc1)-c1ccncc1