Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
Ligand
BDBM50126335
Substrate
n/a
Meas. Tech.
ChEMBL_310131 (CHEMBL838112)
EC50
1.6±n/a nM
Citation
 Li, Q; Claiborne, A; Li, T; Hasvold, L; Stoll, VS; Muchmore, S; Jakob, CG; Gu, W; Cohen, J; Hutchins, C; Frost, D; Rosenberg, SH; Sham, HL Design, synthesis, and activity of 4-quinolone and pyridone compounds as nonthiol-containing farnesyltransferase inhibitors. Bioorg Med Chem Lett 14:5367-70 (2004) [PubMed] Article
Li, Q; Claiborne, A; Li, T; Hasvold, L; Stoll, VS; Muchmore, S; Jakob, CG; Gu, W; Cohen, J; Hutchins, C; Frost, D; Rosenberg, SH; Sham, HL Design, synthesis, and activity of 4-quinolone and pyridone compounds as nonthiol-containing farnesyltransferase inhibitors. Bioorg Med Chem Lett 14:5367-70 (2004) [PubMed] Article More Info.:
Target
Name:
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
Synonyms:
Protein farnesyltransferase | Protein farnesyltransferase beta/geranylgeranyltransferase type I alpha subunit
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of EBI is 38702
Components:
This complex has 2 components.
Component 1
Name:
Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
Synonyms:
CAAX farnesyltransferase subunit alpha | FNTA_MOUSE | FTase-alpha | Fnta | GGTase-I-alpha | Geranylgeranyl transferase type 1 | Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha | Ras proteins prenyltransferase subunit alpha | Type I protein geranyl-geranyltransferase subunit alpha
Type:
PROTEIN
Mol. Mass.:
43994.09
Organism:
Mus musculus
Description:
EBI_10570
Residue:
377
Sequence:
MAATEGVGESAAGGEPGQPEQPPPPPPPPPAQQPQEEEMAAEAGEAAASPMDDGFLSLDSPTYVLYRDRAEWADIDPVPQNDGPNPVVQIIYSEKFRDVYDYFRAVLQRDERSERAFKLTRDAIELNAANYTVWHFRRVLLRSLQKDLQEEMNYITAIIEEQPKNYQVWHHRRVLVEWLKDPSQELEFIADILSQDAKNYHAWQHRQWVIQEFRLWDNELQYVDQLLKEDVRNNSVWNQRHFVISNTTGYSDRAVLEREVQYTLEMIKLVPHNESAWNYLKGILQDRGLSRYPNLLNQLLDLQPSHSSPYLIAFLVDVYEDMLENQCDNKEDILNKALELCEILAKEKDTIRKEYWRYIGRSLQSKHCRESDIPASV
Component 2
Name:
Protein farnesyltransferase subunit beta
Synonyms:
FNTB_MOUSE | Fntb | Protein farnesyltransferase
Type:
PROTEIN
Mol. Mass.:
48811.61
Organism:
Mus musculus
Description:
EBI_12341
Residue:
437
Sequence:
MASSSSFTYYCPPSSSPVWSEPLYSLRPEHVRERLQDDSVETVTSIEQAKVEEKIQEVFSSYKFNHLVPRLILQREKHFHYLKRGLRQLTDAYECLDASRPWLCYWILHSLELLDEPIPQIVATDVCQFLELCQSPDGGFGGGPGQYPHLAPTYAAVNALCIIGTEEAYNVINREKLLQYLYSLKQPDGSFLMHVGGEVDVRSAYCAASVASLTNIITPDLFEGTAEWIARCQNWEGGIGGVPGMEAHGGYTFCGLAALVILKKERSLNLKSLLQWVTSRQMRFEGGFQGRCNKLVDGCYSFWQAGLLPLLHRALHAQGDPALSMSHWMFHQQALQEYILMCCQCPAGGLLDKPGKSRDFYHTCYCLSGLSIAQHFGSGAMLHDMVMGVPENVLQPTHPVYNIGPEKVIQATTHFLQKPVPGFEECEDEVTSDPATD
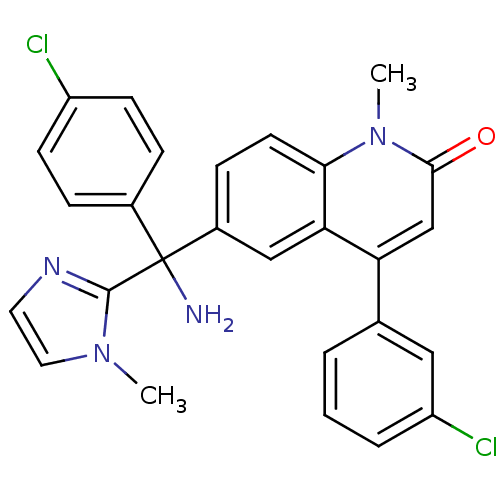
Inhibitor
Name:
BDBM50126335
Synonyms:
6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4-yl)-methyl]-4-(3-chloro-phenyl)-1-methyl-1H-quinolin-2-one | 6-[(R)-Amino-(4-chloro-phenyl)-(3-methyl-4,5-dihydro-3H-imidazol-4-yl)-methyl]-4-(3-chloro-phenyl)-1-methyl-1H-quinolin-2-one | 6-[(S)-AMINO(4-CHLOROPHENYL)(1-METHYL-1H-IMIDAZOL-5-YL)METHYL]-4-(3-CHLOROPHENYL)-1-METHYLQUINOLIN-2(1H)-ONE | 6-[Amino-(4-chloro-phenyl)-(1-methyl-1H-imidazol-2-yl)-methyl]-4-(3-chloro-phenyl)-1-methyl-1H-quinolin-2-one | 6-[Amino-(4-chloro-phenyl)-(3-methyl-3H-imidazol-4-yl)-methyl]-4-(3-chloro-phenyl)-1-methyl-1H-quinolin-2-one | CHEMBL289228 | R-11577 | R-115777 | Tipifarnib
Type:
Small organic molecule
Emp. Form.:
C27H22Cl2N4O
Mol. Mass.:
489.396
SMILES:
Cn1ccnc1C(N)(c1ccc(Cl)cc1)c1ccc2n(C)c(=O)cc(-c3cccc(Cl)c3)c2c1