Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sodium/potassium-transporting ATPase subunit alpha-1
Ligand
BDBM50161244
Substrate
n/a
Meas. Tech.
ChEMBL_304671 (CHEMBL827871)
IC50
1888±n/a nM
Citation
 Van Quaquebeke, E; Simon, G; André, A; Dewelle, J; El Yazidi, M; Bruyneel, F; Tuti, J; Nacoulma, O; Guissou, P; Decaestecker, C; Braekman, JC; Kiss, R; Darro, F Identification of a novel cardenolide (2''-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem 48:849-56 (2005) [PubMed] Article
Van Quaquebeke, E; Simon, G; André, A; Dewelle, J; El Yazidi, M; Bruyneel, F; Tuti, J; Nacoulma, O; Guissou, P; Decaestecker, C; Braekman, JC; Kiss, R; Darro, F Identification of a novel cardenolide (2''-oxovoruscharin) from Calotropis procera and the hemisynthesis of novel derivatives displaying potent in vitro antitumor activities and high in vivo tolerance: structure-activity relationship analyses. J Med Chem 48:849-56 (2005) [PubMed] Article More Info.:
Target
Name:
Sodium/potassium-transporting ATPase subunit alpha-1
Synonyms:
AT1A1_PIG | ATP1A1 | Sodium/potassium-transporting ATPase alpha-1 chain
Type:
PROTEIN
Mol. Mass.:
112664.46
Organism:
Sus scrofa
Description:
ChEMBL_304671
Residue:
1021
Sequence:
MGKGVGRDKYEPAAVSEHGDKKKAKKERDMDELKKEVSMDDHKLSLDELHRKYGTDLSRGLTPARAAEILARDGPNALTPPPTTPEWVKFCRQLFGGFSMLLWIGAILCFLAYGIQAATEEEPQNDNLYLGVVLSAVVIITGCFSYYQEAKSSKIMESFKNMVPQQALVIRNGEKMSINAEEVVVGDLVEVKGGDRIPADLRIISANGCKVDNSSLTGESEPQTRSPDFTNENPLETRNIAFFSTNCVEGTARGIVVYTGDRTVMGRIATLASGLEGGQTPIAAEIEHFIHIITGVAVFLGVSFFILSLILEYTWLEAVIFLIGIIVANVPEGLLATVTVCLTLTAKRMARKNCLVKNLEAVETLGSTSTICSDKTGTLTQNRMTVAHMWSDNQIHEADTTENQSGVSFDKTSATWLALSRIAGLCNRAVFQANQENLPILKRAVAGDASESALLKCIELCCGSVKEMRERYTKIVEIPFNSTNKYQLSIHKNPNTAEPRHLLVMKGAPERILDRCSSILIHGKEQPLDEELKDAFQNAYLELGGLGERVLGFCHLFLPDEQFPEGFQFDTDDVNFPLDNLCFVGLISMIDPPRAAVPDAVGKCRSAGIKVIMVTGDHPITAKAIAKGVGIISEGNETVEDIAARLNIPVSQVNPRDAKACVVHGSDLKDMTSEQLDDILKYHTEIVFARTSPQQKLIIVEGCQRQGAIVAVTGDGVNDSPASKKADIGVAMGIAGSDVSKQAADMILLDDNFASIVTGVEEGRLIFDNLKKSIAYTLTSNIPEITPFLIFIIANIPLPLGTVTILCIDLGTDMVPAISLAYEQAESDIMKRQPRNPKTDKLVNEQLISMAYGQIGMIQALGGFFTYFVILAENGFLPIHLLGLRVNWDDRWINDVEDSYGQQWTYEQRKIVEFTCHTPFFVTIVVVQWADLVICKTRRNSVFQQGMKNKILIFGLFEETALAAFLSYCPGMGVALRMYPLKPTWWFCAFPYSLLIFVYDEVRKLIIRRRPGGWVEKETYY
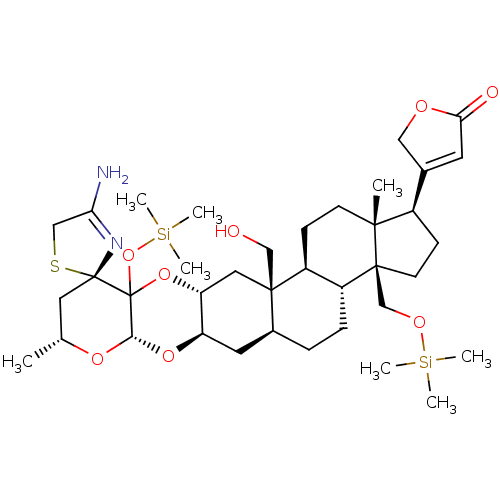
Inhibitor
Name:
BDBM50161244
Synonyms:
CHEMBL369515 | Uscharin derivative
Type:
Small organic molecule
Emp. Form.:
C38H62N2O8SSi2
Mol. Mass.:
763.143
SMILES:
C[C@@H]1C[C@]2(SCC(N)=N2)C2(O[Si](C)(C)C)O[C@@H]3C[C@@]4(CO)[C@@H](CC[C@@H]5[C@@H]4CC[C@]4(C)[C@H](CC[C@]54CO[Si](C)(C)C)C4=CC(=O)OC4)C[C@H]3O[C@@H]2O1 |c:7,t:44|