Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mitogen-activated protein kinase 9
Ligand
BDBM50194671
Substrate
n/a
Meas. Tech.
ChEMBL_396652 (CHEMBL871545)
IC50
>25000±n/a nM
Citation
 DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Buchanan, JL; Buckner, WH; Cee, VJ; Chai, L; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Geuns-Meyer, SD; Gore, A; Gu, Y; Henkle, B; Hodous, BL; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; Masse, CE; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; White, RD; Zhao, H; Zhu, L; Zhu, X Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: synthesis, SAR, and in vivo anti-inflammatory activity. J Med Chem 49:5671-86 (2006) [PubMed] Article
DiMauro, EF; Newcomb, J; Nunes, JJ; Bemis, JE; Boucher, C; Buchanan, JL; Buckner, WH; Cee, VJ; Chai, L; Deak, HL; Epstein, LF; Faust, T; Gallant, P; Geuns-Meyer, SD; Gore, A; Gu, Y; Henkle, B; Hodous, BL; Hsieh, F; Huang, X; Kim, JL; Lee, JH; Martin, MW; Masse, CE; McGowan, DC; Metz, D; Mohn, D; Morgenstern, KA; Oliveira-dos-Santos, A; Patel, VF; Powers, D; Rose, PE; Schneider, S; Tomlinson, SA; Tudor, YY; Turci, SM; Welcher, AA; White, RD; Zhao, H; Zhu, L; Zhu, X Discovery of aminoquinazolines as potent, orally bioavailable inhibitors of Lck: synthesis, SAR, and in vivo anti-inflammatory activity. J Med Chem 49:5671-86 (2006) [PubMed] Article More Info.:
Target
Name:
Mitogen-activated protein kinase 9
Synonyms:
JNK-55 | JNK2 | JNK2/JNK3 | MAPK9 | MK09_HUMAN | Mitogen-Activated Protein Kinase 9 (JNK2) | Mitogen-activated protein kinase 8/9 | PRKM9 | SAPK1A | Stress-activated protein kinase JNK2 | c-Jun N-terminal kinase 2 | c-Jun N-terminal kinase 2 (JNK2)
Type:
Enzyme
Mol. Mass.:
48131.49
Organism:
Homo sapiens (Human)
Description:
JNK-2 was purchased from Upstate Cell Signaling Solutions (formerly Upstate Biotechnology).
Residue:
424
Sequence:
MSDSKCDSQFYSVQVADSTFTVLKRYQQLKPIGSGAQGIVCAAFDTVLGINVAVKKLSRPFQNQTHAKRAYRELVLLKCVNHKNIISLLNVFTPQKTLEEFQDVYLVMELMDANLCQVIHMELDHERMSYLLYQMLCGIKHLHSAGIIHRDLKPSNIVVKSDCTLKILDFGLARTACTNFMMTPYVVTRYYRAPEVILGMGYKENVDIWSVGCIMGELVKGCVIFQGTDHIDQWNKVIEQLGTPSAEFMKKLQPTVRNYVENRPKYPGIKFEELFPDWIFPSESERDKIKTSQARDLLSKMLVIDPDKRISVDEALRHPYITVWYDPAEAEAPPPQIYDAQLEEREHAIEEWKELIYKEVMDWEERSKNGVVKDQPSDAAVSSNATPSQSSSINDISSMSTEQTLASDTDSSLDASTGPLEGCR
Inhibitor
Name:
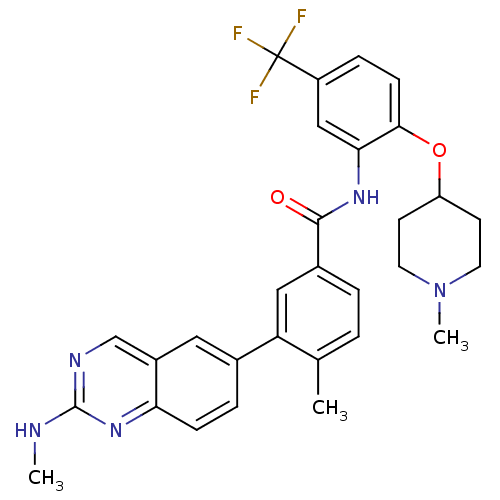
BDBM50194671
Synonyms:
4-methyl-3-(2-(methylamino)quinazolin-6-yl)-N-(2-(1-methylpiperidin-4-yloxy)-5-(trifluoromethyl)phenyl)benzamide | CHEMBL386661
Type:
Small organic molecule
Emp. Form.:
C30H30F3N5O2
Mol. Mass.:
549.5867
SMILES:
CNc1ncc2cc(ccc2n1)-c1cc(ccc1C)C(=O)Nc1cc(ccc1OC1CCN(C)CC1)C(F)(F)F