Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50220969
Substrate
n/a
Meas. Tech.
ChEMBL_457444 (CHEMBL941016)
IC50
5600±n/a nM
Citation
 Garbaccio, RM; Tasber, ES; Neilson, LA; Coleman, PJ; Fraley, ME; Olson, C; Bergman, J; Torrent, M; Buser, CA; Rickert, K; Walsh, ES; Hamilton, K; Lobell, RB; Tao, W; South, VJ; Diehl, RE; Davide, JP; Yan, Y; Kuo, LC; Li, C; Prueksaritanont, T; Fernandez-Metzler, C; Mahan, EA; Slaughter, DE; Salata, JJ; Kohl, NE; Huber, HE; Hartman, GD Kinesin spindle protein (KSP) inhibitors. Part 7: Design and synthesis of 3,3-disubstituted dihydropyrazolobenzoxazines as potent inhibitors of the mitotic kinesin KSP. Bioorg Med Chem Lett 17:5671-6 (2007) [PubMed] Article
Garbaccio, RM; Tasber, ES; Neilson, LA; Coleman, PJ; Fraley, ME; Olson, C; Bergman, J; Torrent, M; Buser, CA; Rickert, K; Walsh, ES; Hamilton, K; Lobell, RB; Tao, W; South, VJ; Diehl, RE; Davide, JP; Yan, Y; Kuo, LC; Li, C; Prueksaritanont, T; Fernandez-Metzler, C; Mahan, EA; Slaughter, DE; Salata, JJ; Kohl, NE; Huber, HE; Hartman, GD Kinesin spindle protein (KSP) inhibitors. Part 7: Design and synthesis of 3,3-disubstituted dihydropyrazolobenzoxazines as potent inhibitors of the mitotic kinesin KSP. Bioorg Med Chem Lett 17:5671-6 (2007) [PubMed] Article More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
Name:
BDBM50220969
Synonyms:
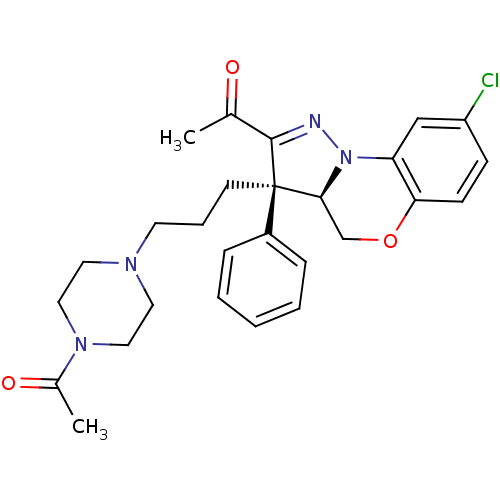
1-{(3R,3aR)-3-[3-(4-acetyl-piperazin-1-yl)-propyl]-8-chloro-3-phenyl-3a,4-dihydro-3H-5-oxa-1,9b-diaza-cyclopenta[a]naphthalen-2-yl}-ethanone | CHEMBL250126
Type:
Small organic molecule
Emp. Form.:
C27H31ClN4O3
Mol. Mass.:
495.013
SMILES:
CC(=O)N1CCN(CCC[C@]2([C@@H]3COc4ccc(Cl)cc4N3N=C2C(C)=O)c2ccccc2)CC1 |c:24|