Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Lactoylglutathione lyase
Ligand
BDBM15236
Substrate
n/a
Meas. Tech.
ChEMBL_529831 (CHEMBL967534)
IC50
560±n/a nM
Citation
 Takasawa, R; Takahashi, S; Saeki, K; Sunaga, S; Yoshimori, A; Tanuma, S Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg Med Chem 16:3969-75 (2008) [PubMed] Article
Takasawa, R; Takahashi, S; Saeki, K; Sunaga, S; Yoshimori, A; Tanuma, S Structure-activity relationship of human GLO I inhibitory natural flavonoids and their growth inhibitory effects. Bioorg Med Chem 16:3969-75 (2008) [PubMed] Article More Info.:
Target
Name:
Lactoylglutathione lyase
Synonyms:
Aldoketomutase | GLO1 | Glx I | Glyoxalase 1 (GLO1) | Glyoxalase I | Ketone-aldehyde mutase | LGUL_HUMAN | Methylglyoxalase | S-D-lactoylglutathione methylglyoxal lyase
Type:
Enzyme
Mol. Mass.:
20772.95
Organism:
Homo sapiens (Human)
Description:
Q04760
Residue:
184
Sequence:
MAEPQPPSGGLTDEAALSCCSDADPSTKDFLLQQTMLRVKDPKKSLDFYTRVLGMTLIQKCDFPIMKFSLYFLAYEDKNDIPKEKDEKIAWALSRKATLELTHNWGTEDDETQSYHNGNSDPRGFGHIGIAVPDVYSACKRFEELGVKFVKKPDDGKMKGLAFIQDPDGYWIEILNPNKMATLM
Inhibitor
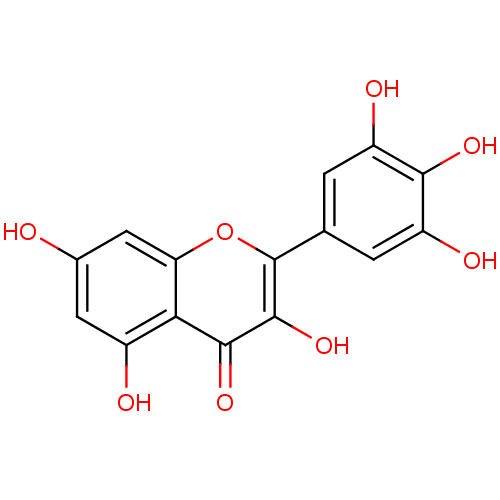
Name:
BDBM15236
Synonyms:
3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one | 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | CHEMBL164 | Cannabiscetin | Myricetin | Myricetin (20) | Myricetin (Myr) | cid_5281672
Type:
Small organic molecule
Emp. Form.:
C15H10O8
Mol. Mass.:
318.2351
SMILES:
Oc1cc(O)c2c(c1)oc(-c1cc(O)c(O)c(O)c1)c(O)c2=O