Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Trypanothione reductase
Ligand
BDBM50170726
Substrate
n/a
Meas. Tech.
ChEMBL_571029 (CHEMBL1024385)
Ki
16000±n/a nM
Citation
 Cavalli, A; Lizzi, F; Bongarzone, S; Brun, R; Luise Krauth-Siegel, R; Bolognesi, ML Privileged structure-guided synthesis of quinazoline derivatives as inhibitors of trypanothione reductase. Bioorg Med Chem Lett 19:3031-5 (2009) [PubMed] Article
Cavalli, A; Lizzi, F; Bongarzone, S; Brun, R; Luise Krauth-Siegel, R; Bolognesi, ML Privileged structure-guided synthesis of quinazoline derivatives as inhibitors of trypanothione reductase. Bioorg Med Chem Lett 19:3031-5 (2009) [PubMed] Article More Info.:
Target
Name:
Trypanothione reductase
Synonyms:
N(1),N(8)-bis(glutathionyl)spermidine reductase | TPR | TR | TYTR_TRYCR
Type:
Homodimer; oxidoreductase
Mol. Mass.:
53868.26
Organism:
Trypanosoma cruzi
Description:
n/a
Residue:
492
Sequence:
MMSKIFDLVVIGAGSGGLEAAWNAATLYKKRVAVIDVQMVHGPPFFSALGGTCVNVGCVPKKLMVTGAQYMEHLRESAGFGWEFDRTTLRAEWKKLIAVKDEAVLNINKSYEEMFRDTEGLEFFLGWGSLESKNVVNVRESADPASAVKERLETENILLASGSWPHMPNIPGIEHCISSNEAFYLPEPPRRVLTVGGGFISVEFAGIFNAYKPKDGQVTLCYRGEMILRGFDHTLREELTKQLTANGIQILTKENPAKVELNADGSKSVTFESGKKMDFDLVMMAIGRSPRTKDLQLQNAGVMIKNGGVQVDEYSRTNVSNIYAIGDVTNRVMLTPVAINEAAALVDTVFGTNPRKTDHTRVASAVFSIPPIGTCGLIEEVASKRYEVVAVYLSSFTPLMHNISGSKYKTFVAKIITNHSDGTVLGVHLLGDNAPEIIQGVGICLKLNAKISDFYNTIGVHPTSAEELCSMRTPSYYYVKGEKMEKPSEASL
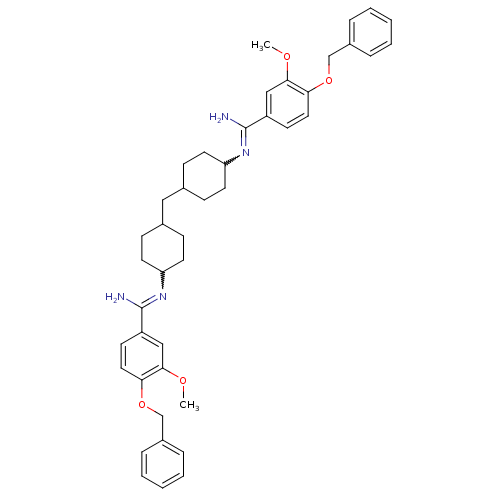
Inhibitor
Name:
BDBM50170726
Synonyms:
4,4'-Bis(4-benzyloxy-3-methoxybenzimidoylamino)dicyclohexylmethane | 4,4'-bis(4-benzyloxy-3-methoxybenzimidoylamino)di-cyclohexylmethane | CHEMBL188765
Type:
Small organic molecule
Emp. Form.:
C43H52N4O4
Mol. Mass.:
688.8974
SMILES:
COc1cc(ccc1OCc1ccccc1)C(N)=NC1CCC(CC2CCC(CC2)N=C(N)c2ccc(OCc3ccccc3)c(OC)c2)CC1 |w:18.20,30.32,(-1.23,-7.92,;.33,-7.9,;1.12,-9.21,;2.66,-9.19,;3.45,-10.52,;2.7,-11.87,;1.15,-11.89,;.36,-10.56,;-1.16,-10.59,;-1.93,-11.94,;-3.47,-11.96,;-4.22,-13.3,;-5.75,-13.33,;-6.55,-12,;-5.79,-10.66,;-4.28,-10.63,;4.9,-11,;5.23,-12.5,;6.06,-9.97,;7.51,-10.45,;8.65,-9.42,;10.1,-9.89,;10.42,-11.4,;11.89,-11.87,;13.03,-10.84,;14.5,-11.33,;15.64,-10.3,;15.32,-8.79,;13.85,-8.33,;12.71,-9.35,;16.46,-7.77,;17.92,-8.23,;18.25,-9.74,;18.67,-6.88,;20.21,-6.83,;20.95,-5.48,;20.14,-4.16,;20.86,-2.8,;22.4,-2.73,;23.12,-1.38,;22.3,-.08,;23.02,1.3,;24.56,1.37,;25.38,.04,;24.66,-1.33,;18.59,-4.22,;17.78,-2.92,;18.5,-1.57,;17.87,-5.55,;9.28,-12.43,;7.83,-11.95,)|