Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leukotriene B4 receptor 1
Ligand
BDBM50317643
Substrate
n/a
Meas. Tech.
ChEMBL_632889 (CHEMBL1107471)
IC50
5.39±n/a nM
Citation
 Goodnow, RA; Hicks, A; Sidduri, A; Kowalczyk, A; Dominique, R; Qiao, Q; Lou, JP; Gillespie, P; Fotouhi, N; Tilley, J; Cohen, N; Choudhry, S; Cavallo, G; Tannu, SA; Ventre, JD; Lavelle, D; Tare, NS; Oh, H; Lamb, M; Kurylko, G; Hamid, R; Wright, MB; Pamidimukkala, A; Egan, T; Gubler, U; Hoffman, AF; Wei, X; Li, YL; O'Neil, J; Marcano, R; Pozzani, K; Molinaro, T; Santiago, J; Singer, L; Hargaden, M; Moore, D; Catala, AR; Chao, LC; Hermann, G; Venkat, R; Mancebo, H; Renzetti, LM Discovery of novel and potent leukotriene B4 receptor antagonists. Part 1. J Med Chem 53:3502-16 (2010) [PubMed] Article
Goodnow, RA; Hicks, A; Sidduri, A; Kowalczyk, A; Dominique, R; Qiao, Q; Lou, JP; Gillespie, P; Fotouhi, N; Tilley, J; Cohen, N; Choudhry, S; Cavallo, G; Tannu, SA; Ventre, JD; Lavelle, D; Tare, NS; Oh, H; Lamb, M; Kurylko, G; Hamid, R; Wright, MB; Pamidimukkala, A; Egan, T; Gubler, U; Hoffman, AF; Wei, X; Li, YL; O'Neil, J; Marcano, R; Pozzani, K; Molinaro, T; Santiago, J; Singer, L; Hargaden, M; Moore, D; Catala, AR; Chao, LC; Hermann, G; Venkat, R; Mancebo, H; Renzetti, LM Discovery of novel and potent leukotriene B4 receptor antagonists. Part 1. J Med Chem 53:3502-16 (2010) [PubMed] Article More Info.:
Target
Name:
Leukotriene B4 receptor 1
Synonyms:
BLT | BLT1 | BLTR | CMKRL1 | Chemoattractant receptor-like 1 | G-protein coupled receptor 16 | GPR16 | LT4R1_HUMAN | LTB4-R 1 | LTB4R | Leukotriene B4 R1 | Leukotriene B4 receptor | Leukotriene B4 receptor 1 | P2RY7 | P2Y purinoceptor 7 | P2Y7
Type:
Enzyme Catalytic Domain
Mol. Mass.:
37582.68
Organism:
Homo sapiens (Human)
Description:
Q15722
Residue:
352
Sequence:
MNTTSSAAPPSLGVEFISLLAIILLSVALAVGLPGNSFVVWSILKRMQKRSVTALMVLNLALADLAVLLTAPFFLHFLAQGTWSFGLAGCRLCHYVCGVSMYASVLLITAMSLDRSLAVARPFVSQKLRTKAMARRVLAGIWVLSFLLATPVLAYRTVVPWKTNMSLCFPRYPSEGHRAFHLIFEAVTGFLLPFLAVVASYSDIGRRLQARRFRRSRRTGRLVVLIILTFAAFWLPYHVVNLAEAGRALAGQAAGLGLVGKRLSLARNVLIALAFLSSSVNPVLYACAGGGLLRSAGVGFVAKLLEGTGSEASSTRRGGSLGQTARSGPAALEPGPSESLTASSPLKLNELN
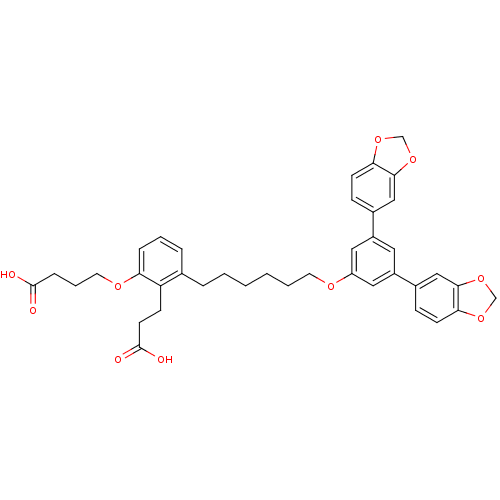
Inhibitor
Name:
BDBM50317643
Synonyms:
4-[3-[6-(3,5-Bis-benzo[1,3]dioxol-5-yl-phenoxy)-hexyl]-2-(2-carboxy-ethyl)-phenoxy]-butyric acid | CHEMBL1099328
Type:
Small organic molecule
Emp. Form.:
C39H40O10
Mol. Mass.:
668.7289
SMILES:
OC(=O)CCCOc1cccc(CCCCCCOc2cc(cc(c2)-c2ccc3OCOc3c2)-c2ccc3OCOc3c2)c1CCC(O)=O