Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
Ligand
BDBM50343200
Substrate
n/a
Meas. Tech.
ChEMBL_745550 (CHEMBL1775867)
Ki
2±n/a nM
Citation
 D'Angelo, ND; Kim, TS; Andrews, K; Booker, SK; Caenepeel, S; Chen, K; D'Amico, D; Freeman, D; Jiang, J; Liu, L; McCarter, JD; San Miguel, T; Mullady, EL; Schrag, M; Subramanian, R; Tang, J; Wahl, RC; Wang, L; Whittington, DA; Wu, T; Xi, N; Xu, Y; Yakowec, P; Yang, K; Zalameda, LP; Zhang, N; Hughes, P; Norman, MH Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem 54:1789-811 (2011) [PubMed] Article
D'Angelo, ND; Kim, TS; Andrews, K; Booker, SK; Caenepeel, S; Chen, K; D'Amico, D; Freeman, D; Jiang, J; Liu, L; McCarter, JD; San Miguel, T; Mullady, EL; Schrag, M; Subramanian, R; Tang, J; Wahl, RC; Wang, L; Whittington, DA; Wu, T; Xi, N; Xu, Y; Yakowec, P; Yang, K; Zalameda, LP; Zhang, N; Hughes, P; Norman, MH Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem 54:1789-811 (2011) [PubMed] Article More Info.:
Target
Name:
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
Synonyms:
PI3-kinase p110 subunit beta | PI3-kinase subunit p110-beta | PI3Kbeta | PIK3C1 | PIK3CB | PK3CB_HUMAN | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3K-beta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kbeta) | Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PI3Kÿ²) | Phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta isoform | Phosphoinositide 3-Kinase (PI3K), beta | Phosphoinositide 3-Kinase (PI3K), beta Chain A | Phosphoinositide-3-kinase (PI3K beta) | PtdIns-3-kinase p110
Type:
Enzyme Subunit
Mol. Mass.:
122769.00
Organism:
Homo sapiens (Human)
Description:
P42338
Residue:
1070
Sequence:
MCFSFIMPPAMADILDIWAVDSQIASDGSIPVDFLLPTGIYIQLEVPREATISYIKQMLWKQVHNYPMFNLLMDIDSYMFACVNQTAVYEELEDETRRLCDVRPFLPVLKLVTRSCDPGEKLDSKIGVLIGKGLHEFDSLKDPEVNEFRRKMRKFSEEKILSLVGLSWMDWLKQTYPPEHEPSIPENLEDKLYGGKLIVAVHFENCQDVFSFQVSPNMNPIKVNELAIQKRLTIHGKEDEVSPYDYVLQVSGRVEYVFGDHPLIQFQYIRNCVMNRALPHFILVECCKIKKMYEQEMIAIEAAINRNSSNLPLPLPPKKTRIISHVWENNNPFQIVLVKGNKLNTEETVKVHVRAGLFHGTELLCKTIVSSEVSGKNDHIWNEPLEFDINICDLPRMARLCFAVYAVLDKVKTKKSTKTINPSKYQTIRKAGKVHYPVAWVNTMVFDFKGQLRTGDIILHSWSSFPDELEEMLNPMGTVQTNPYTENATALHVKFPENKKQPYYYPPFDKIIEKAAEIASSDSANVSSRGGKKFLPVLKEILDRDPLSQLCENEMDLIWTLRQDCREIFPQSLPKLLLSIKWNKLEDVAQLQALLQIWPKLPPREALELLDFNYPDQYVREYAVGCLRQMSDEELSQYLLQLVQVLKYEPFLDCALSRFLLERALGNRRIGQFLFWHLRSEVHIPAVSVQFGVILEAYCRGSVGHMKVLSKQVEALNKLKTLNSLIKLNAVKLNRAKGKEAMHTCLKQSAYREALSDLQSPLNPCVILSELYVEKCKYMDSKMKPLWLVYNNKVFGEDSVGVIFKNGDDLRQDMLTLQMLRLMDLLWKEAGLDLRMLPYGCLATGDRSGLIEVVSTSETIADIQLNSSNVAAAAAFNKDALLNWLKEYNSGDDLDRAIEEFTLSCAGYCVASYVLGIGDRHSDNIMVKKTGQLFHIDFGHILGNFKSKFGIKRERVPFILTYDFIHVIQQGKTGNTEKFGRFRQCCEDAYLILRRHGNLFITLFALMLTAGLPELTSVKDIQYLKDSLALGKSEEEALKQFKQKFDEALRESWTTKVNWMAHTVRKDYRS
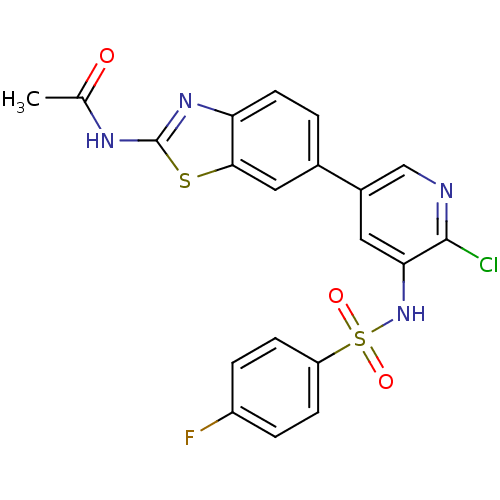
Inhibitor
Name:
BDBM50343200
Synonyms:
CHEMBL1615189 | N-(6-(6-Chloro-5-(4-fluorophenylsulfonamido)pyridin-3-yl)benzo[d]thiazol-2-yl)acetamide | N-[6-(6-chloro-5-{[(4-fluorophenyl)sulfonyl]amino}pyridin-3-yl)-1,3-benzothiazol-2-yl]acetamide
Type:
Small organic molecule
Emp. Form.:
C20H14ClFN4O3S2
Mol. Mass.:
476.932
SMILES:
CC(=O)Nc1nc2ccc(cc2s1)-c1cnc(Cl)c(NS(=O)(=O)c2ccc(F)cc2)c1