Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Glucocorticoid receptor
Ligand
BDBM19190
Substrate
n/a
Meas. Tech.
ChEMBL_776318 (CHEMBL1914169)
EC50
21±n/a nM
Citation
 Weinstein, DS; Gong, H; Doweyko, AM; Cunningham, M; Habte, S; Wang, JH; Holloway, DA; Burke, C; Gao, L; Guarino, V; Carman, J; Somerville, JE; Shuster, D; Salter-Cid, L; Dodd, JH; Nadler, SG; Barrish, JC Azaxanthene based selective glucocorticoid receptor modulators: design, synthesis, and pharmacological evaluation of (S)-4-(5-(1-((1,3,4-thiadiazol-2-yl)amino)-2-methyl-1-oxopropan-2-yl)-5H-chromeno[2,3-b]pyridin-2-yl)-2-fluoro-N,N-dimethylbenzamide (BMS-776532) and its methylene homologue (BMS-791 J Med Chem 54:7318-33 (2011) [PubMed] Article
Weinstein, DS; Gong, H; Doweyko, AM; Cunningham, M; Habte, S; Wang, JH; Holloway, DA; Burke, C; Gao, L; Guarino, V; Carman, J; Somerville, JE; Shuster, D; Salter-Cid, L; Dodd, JH; Nadler, SG; Barrish, JC Azaxanthene based selective glucocorticoid receptor modulators: design, synthesis, and pharmacological evaluation of (S)-4-(5-(1-((1,3,4-thiadiazol-2-yl)amino)-2-methyl-1-oxopropan-2-yl)-5H-chromeno[2,3-b]pyridin-2-yl)-2-fluoro-N,N-dimethylbenzamide (BMS-776532) and its methylene homologue (BMS-791 J Med Chem 54:7318-33 (2011) [PubMed] Article More Info.:
Target
Name:
Glucocorticoid receptor
Synonyms:
GCR_HUMAN | GR | GRL | Glucocorticoid | Glucocorticoid receptor (GRFP) | NR3C1 | Nuclear receptor subfamily 3 group C member 1
Type:
Enzyme
Mol. Mass.:
85656.87
Organism:
Homo sapiens (Human)
Description:
P04150
Residue:
777
Sequence:
MDSKESLTPGREENPSSVLAQERGDVMDFYKTLRGGATVKVSASSPSLAVASQSDSKQRRLLVDFPKGSVSNAQQPDLSKAVSLSMGLYMGETETKVMGNDLGFPQQGQISLSSGETDLKLLEESIANLNRSTSVPENPKSSASTAVSAAPTEKEFPKTHSDVSSEQQHLKGQTGTNGGNVKLYTTDQSTFDILQDLEFSSGSPGKETNESPWRSDLLIDENCLLSPLAGEDDSFLLEGNSNEDCKPLILPDTKPKIKDNGDLVLSSPSNVTLPQVKTEKEDFIELCTPGVIKQEKLGTVYCQASFPGANIIGNKMSAISVHGVSTSGGQMYHYDMNTASLSQQQDQKPIFNVIPPIPVGSENWNRCQGSGDDNLTSLGTLNFPGRTVFSNGYSSPSMRPDVSSPPSSSSTATTGPPPKLCLVCSDEASGCHYGVLTCGSCKVFFKRAVEGQHNYLCAGRNDCIIDKIRRKNCPACRYRKCLQAGMNLEARKTKKKIKGIQQATTGVSQETSENPGNKTIVPATLPQLTPTLVSLLEVIEPEVLYAGYDSSVPDSTWRIMTTLNMLGGRQVIAAVKWAKAIPGFRNLHLDDQMTLLQYSWMFLMAFALGWRSYRQSSANLLCFAPDLIINEQRMTLPCMYDQCKHMLYVSSELHRLQVSYEEYLCMKTLLLLSSVPKDGLKSQELFDEIRMTYIKELGKAIVKREGNSSQNWQRFYQLTKLLDSMHEVVENLLNYCFQTFLDKTMSIEFPEMLAEIITNQIPKYSNGNIKKLLFHQK
Inhibitor
Name:
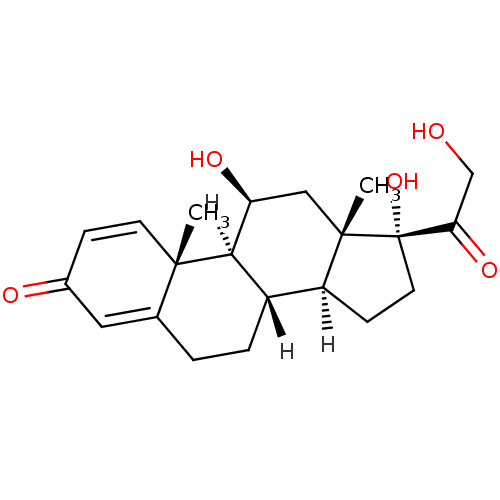
BDBM19190
Synonyms:
(1S,2R,10S,11S,14R,15S,17S)-14,17-dihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-3,6-dien-5-one | CHEMBL131 | Delta-Cortef | Deltacortril | Prednisolone | US10196374, Prednisolone
Type:
Steroid
Emp. Form.:
C21H28O5
Mol. Mass.:
360.444
SMILES:
[H][C@@]12CC[C@](O)(C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]1([H])[C@@]2([H])CCC2=CC(=O)C=C[C@]12C |r,c:27,t:23|