Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 1A2
Ligand
BDBM50131047
Substrate
n/a
Meas. Tech.
ChEBML_51362
IC50
5630±n/a nM
Citation
More Info.:
Target
Name:
Cytochrome P450 1A2
Synonyms:
CP1A2_HUMAN | CYP1A2 | CYPIA2 | Cholesterol 25-hydroxylase | Cytochrome P(3)450 | Cytochrome P450 1A | Cytochrome P450 1A2 (CYP1A2) | Cytochrome P450 4 | Cytochrome P450-P3
Type:
Enzyme
Mol. Mass.:
58423.38
Organism:
Homo sapiens (Human)
Description:
P05177
Residue:
516
Sequence:
MALSQSVPFSATELLLASAIFCLVFWVLKGLRPRVPKGLKSPPEPWGWPLLGHVLTLGKNPHLALSRMSQRYGDVLQIRIGSTPVLVLSRLDTIRQALVRQGDDFKGRPDLYTSTLITDGQSLTFSTDSGPVWAARRRLAQNALNTFSIASDPASSSSCYLEEHVSKEAKALISRLQELMAGPGHFDPYNQVVVSVANVIGAMCFGQHFPESSDEMLSLVKNTHEFVETASSGNPLDFFPILRYLPNPALQRFKAFNQRFLWFLQKTVQEHYQDFDKNSVRDITGALFKHSKKGPRASGNLIPQEKIVNLVNDIFGAGFDTVTTAISWSLMYLVTKPEIQRKIQKELDTVIGRERRPRLSDRPQLPYLEAFILETFRHSSFLPFTIPHSTTRDTTLNGFYIPKKCCVFVNQWQVNHDPELWEDPSEFRPERFLTADGTAINKPLSEKMMLFGMGKRRCIGEVLAKWEIFLFLAILLQQLEFSVPPGVKVDLTPIYGLTMKHARCEHVQARLRFSIN
Inhibitor
Name:
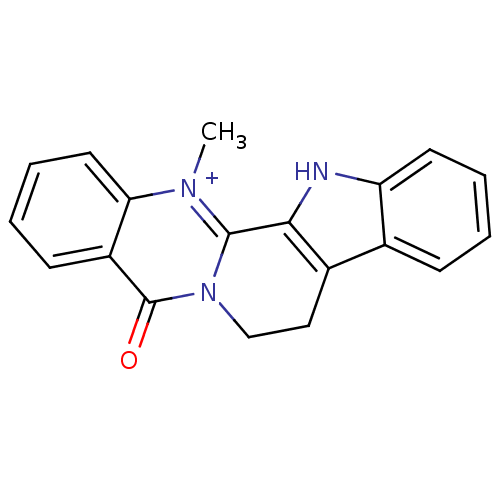
BDBM50131047
Synonyms:
14-Methyl-5-oxo-5,7,8,13-tetrahydro-indolo[2',3':3,4]pyrido[2,1-b]quinazolin-14-ium | CHEMBL81923 | dehydroevidiamine | dehydroevodiamine | dehydroevodiamine chloride | dehydroevodiamine hydrochloride
Type:
Small organic molecule
Emp. Form.:
C19H16N3O
Mol. Mass.:
302.3493
SMILES:
C[n+]1c2-c3[nH]c4ccccc4c3CCn2c(=O)c2ccccc12