Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Leucyl-cystinyl aminopeptidase
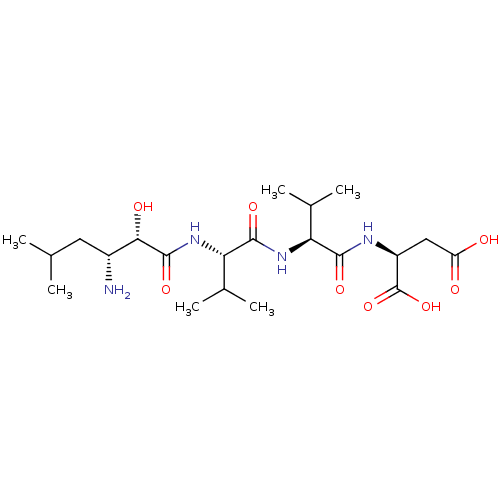
Ligand
BDBM50017478
Substrate
n/a
Meas. Tech.
ChEMBL_98526 (CHEMBL706546)
Ki
220±n/a nM
Citation
 Harbeson, SL; Rich, DH Inhibition of aminopeptidases by peptides containing ketomethylene and hydroxyethylene amide bond replacements. J Med Chem 32:1378-92 (1989) [PubMed] Article
Harbeson, SL; Rich, DH Inhibition of aminopeptidases by peptides containing ketomethylene and hydroxyethylene amide bond replacements. J Med Chem 32:1378-92 (1989) [PubMed] Article More Info.:
Target
Name:
Leucyl-cystinyl aminopeptidase
Synonyms:
Cystinyl aminopeptidase | GP160 | Insulin-regulated membrane aminopeptidase | Insulin-responsive aminopeptidase | Irap | LCAP_RAT | Leucyl-cystinyl aminopeptidase | Lnpep | Otase | Oxytocinase | P-LAP | Placental leucine aminopeptidase | Vesicle protein of 165 kDa | Vp165
Type:
PROTEIN
Mol. Mass.:
117179.39
Organism:
Rattus norvegicus
Description:
ChEMBL_98523
Residue:
1025
Sequence:
METFTNDRLQLPRNMIENSMFEEEPDVVDLAKEPCLHPLEPDEVEYEPRGSRLLVRGLGEHEMDEDEEDYESSAKLLGMSFMNRSSGLRNSATGYRQSPDGTCSVPSARTLVICVFVIVVAVSVIMVIYLLPRCTFTKEGCHKTNQSAELIQPIATNGKVFPWAQIRLPTAIIPQRYELSLHPNLTSMTFRGSVTISLQALQDTRDIILHSTGHNISSVTFMSAVSSQEKQVEILEYPYHEQIAVVAPESLLTGHNYTLKIEYSANISNSYYGFYGITYTDKSNEKKNFAATQFEPLAARSAFPCFDEPAFKATFIIKITRDEHHTALSNMPKKSSVPTEEGLIQDEFSESVKMSTYLVAFIVGEMRNLSQDVNGTLVSVYAVPEKIDQVYHALDTTVKLLEFYQNYFEIQYPLKKLDLVAIPDFEAGAMENWGLLTFREETLLYDNATSSVADRKLVTKIIAHELAHQWFGNLVTMQWWNDLWLNEGFATFMEYFSVEKIFKELNSYEDFLDARFKTMRKDSLNSSHPISSSVQSSEQIEEMFDSLSYFKGASLLLMLKSYLSEDVFQHAIILYLHNHSYAAIQSDDLWDSFNEVTGKTLDVKKMMKTWTLQKGFPLVTVQRKGTELLLQQERFFPSMQPEIQDSDTSHLWHIPISYVTDGRNYSEYRSVSLLDKKSDVINLTEQVQWVKVNTNMTGYYIVHYAHDGWAALINQLKRNPYVLSDKDRANLINNIFELAGLGKVPLQMAFDLIDYLRNETHTAPITEALFQTDLIYNLLEKLGHMDLSSRLVTRVHKLLQNQIQQQTWTDEGTPSMRELRSALLEFACAHSLENCTTMATKLFDGWMASNGTQSLPTDVMTTVFKVGARTEKGWLFLFSMYSSMGSEAEKDKILEALASSADAHKLYWLMKSSLDGDIIRTQKLSLIIRTVGRQFPGHLLAWDFVKENWNKLVHKFHLGSYTIQSIVAGSTHLFSTKTHLSEVQEFFENQSEATLQLRCVQEAFEVIELNIQWMARNLKTLTLWL
Inhibitor
Name:
BDBM50017478
Synonyms:
Amastatin | CHEMBL28650 | Leu[1psi,CHOHCONH]ValValAsp | N-[(2S,3R)-3-amino-2-hydroxy-5-methylhexanoyl]-L-valyl-L-valyl-L-aspartic acid
Type:
Small organic molecule
Emp. Form.:
C21H38N4O8
Mol. Mass.:
474.5484
SMILES:
CC(C)C[C@@H](N)[C@H](O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(O)=O)C(O)=O