Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Phospholipase A2, membrane associated
Ligand
BDBM50250399
Substrate
n/a
Meas. Tech.
ChEMBL_156196 (CHEMBL767139)
IC50
1500000±n/a nM
Citation
 Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem 39:5183-91 (1997) [PubMed] Article
Teshirogi, I; Matsutani, S; Shirahase, K; Fujii, Y; Yoshida, T; Tanaka, K; Ohtani, M Synthesis and phospholipase A2 inhibitory activity of thielocin B3 derivatives. J Med Chem 39:5183-91 (1997) [PubMed] Article More Info.:
Target
Name:
Phospholipase A2, membrane associated
Synonyms:
GIIC sPLA2 | Group IIA phospholipase A2 | NPS-PLA2 | Non-Pancreatic Secretory Phospholipase A2 | Non-pancreatic secretory phospholipase A2 (hnps-PLA2) | PA2GA_HUMAN | PLA2B | PLA2G2A | PLA2L | Phosphatidylcholine 2-acylhydrolase | Phospholipase A2 group IIA | RASF-A
Type:
Hydrolase
Mol. Mass.:
16101.20
Organism:
Homo sapiens (Human)
Description:
The human nps PLA2 was cloned, and expressed in E. coli. There was a refolding process in the purification.
Residue:
144
Sequence:
MKTLLLLAVIMIFGLLQAHGNLVNFHRMIKLTTGKEAALSYGFYGCHCGVGGRGSPKDATDRCCVTHDCCYKRLEKRGCGTKFLSYKFSNSGSRITCAKQDSCRSQLCECDKAAATCFARNKTTYNKKYQYYSNKHCRGSTPRC
Inhibitor
Name:
BDBM50250399
Synonyms:
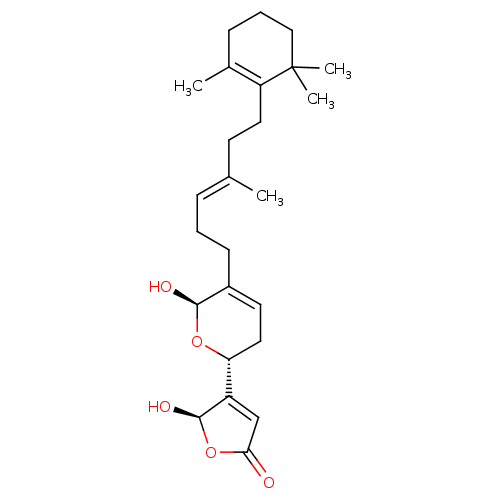
5-Hydroxy-4-{(R)-6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one | 5-Hydroxy-4-{6-hydroxy-5-[(E)-4-methyl-6-(2,6,6-trimethyl-cyclohex-1-enyl)-hex-3-enyl]-3,6-dihydro-2H-pyran-2-yl}-5H-furan-2-one | CHEMBL463914 | manoalide
Type:
Small organic molecule
Emp. Form.:
C25H36O5
Mol. Mass.:
416.5503
SMILES:
C\C(CCC1=C(C)CCCC1(C)C)=C/CCC1=CC[C@@H](O[C@H]1O)C1=CC(=O)O[C@H]1O |r,c:4,t:17,25|