Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
GTPase HRas
Ligand
BDBM50055659
Substrate
n/a
Meas. Tech.
ChEMBL_82664 (CHEMBL694129)
IC50
200±n/a nM
Citation
 Baraldi, PG; Romagnoli, R; Beria, I; Cozzi, P; Geroni, C; Mongelli, N; Bianchi, N; Mischiati, C; Gambari, R Synthesis and antitumor activity of new benzoheterocyclic derivatives of distamycin A. J Med Chem 43:2675-84 (2000) [PubMed] Article
Baraldi, PG; Romagnoli, R; Beria, I; Cozzi, P; Geroni, C; Mongelli, N; Bianchi, N; Mischiati, C; Gambari, R Synthesis and antitumor activity of new benzoheterocyclic derivatives of distamycin A. J Med Chem 43:2675-84 (2000) [PubMed] Article More Info.:
Target
Name:
GTPase HRas
Synonyms:
GTPase HRas, N-terminally processed | H-Ras | H-Ras-1 | HRAS | HRAS1 | Ha-Ras | His6-Ha-Ras-CVLS | RASH_HUMAN | Transforming protein p21 | Transforming protein p21/H-Ras-1 | Wild-type Ha-Ras | c-H-ras | p21ras
Type:
Other Protein Type
Mol. Mass.:
21293.37
Organism:
Homo sapiens (Human)
Description:
P01112
Residue:
189
Sequence:
MTEYKLVVVGAGGVGKSALTIQLIQNHFVDEYDPTIEDSYRKQVVIDGETCLLDILDTAGQEEYSAMRDQYMRTGEGFLCVFAINNTKSFEDIHQYREQIKRVKDSDDVPMVLVGNKCDLAARTVESRQAQDLARSYGIPYIETSAKTRQGVEDAFYTLVREIRQHKLRKLNPPDESGPGCMSCKCVLS
Inhibitor
Name:
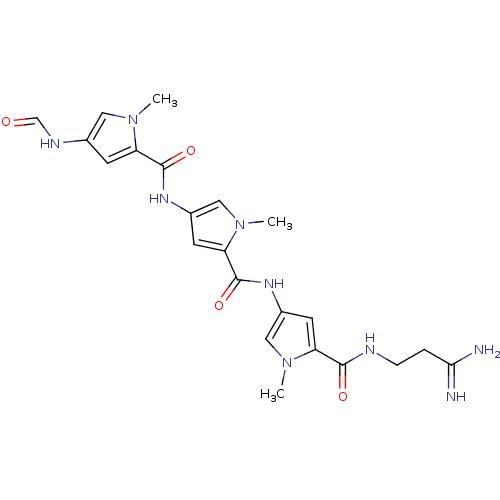
BDBM50055659
Synonyms:
2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolylcarboxamido]-1-methyl-1H-2-pyrrolecarboxamide | 2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide | 2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide(Distamycin) | 2N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-3-pyrrolyl]-4-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-2-pyrrolecarboxamide(distamycin A) | 4N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-1H-2-pyrrolyl]-2-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-1H-4-pyrrolecarboxamide | 5N-[5-(3-amino-3-iminopropylcarbamoyl)-1-methyl-2,3-dihydro-1H-3-pyrrolyl]-3-(4-formamido-1-methyl-1H-2-pyrrolylcarboxamido)-1-methyl-2,3-dihydro-1H-5-pyrrolecarboxamide | CHEMBL101290 | CHEMBL11252 | DISTAMYCIN | DISTAMYCIN HYDROCHLORIDE | cid_6602691
Type:
Small organic molecule
Emp. Form.:
C22H27N9O4
Mol. Mass.:
481.5077
SMILES:
Cn1cc(NC=O)cc1C(=O)Nc1cc(C(=O)Nc2cc(C(=O)NCCC(N)=N)n(C)c2)n(C)c1