Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Aminopeptidase N
Ligand
BDBM50087111
Substrate
n/a
Meas. Tech.
ChEMBL_28885 (CHEMBL646583)
Ki
4.2±n/a nM
Citation
 Chen, H; Noble, F; Mothé, A; Meudal, H; Coric, P; Danascimento, S; Roques, BP; George, P; Fournié-Zaluski, MC Phosphinic derivatives as new dual enkephalin-degrading enzyme inhibitors: synthesis, biological properties, and antinociceptive activities. J Med Chem 43:1398-408 (2001) [PubMed] Article
Chen, H; Noble, F; Mothé, A; Meudal, H; Coric, P; Danascimento, S; Roques, BP; George, P; Fournié-Zaluski, MC Phosphinic derivatives as new dual enkephalin-degrading enzyme inhibitors: synthesis, biological properties, and antinociceptive activities. J Med Chem 43:1398-408 (2001) [PubMed] Article More Info.:
Target
Name:
Aminopeptidase N
Synonyms:
AMPN_PIG | ANPEP | AP-M | AP-N | Alanyl aminopeptidase | Aminopeptidase M | Aminopeptidase N (APN) | CD_antigen=CD13 | Microsomal aminopeptidase | gp130 | pAPN
Type:
Protein
Mol. Mass.:
108810.25
Organism:
Sus scrofa (Pig)
Description:
P15145
Residue:
963
Sequence:
MAKGFYISKALGILGILLGVAAVATIIALSVVYAQEKNKNAEHVPQAPTSPTITTTAAITLDQSKPWNRYRLPTTLLPDSYNVTLRPYLTPNADGLYIFKGKSIVRLLCQEPTDVIIIHSKKLNYTTQGHMVVLRGVGDSQVPEIDRTELVELTEYLVVHLKGSLQPGHMYEMESEFQGELADDLAGFYRSEYMEGNVKKVLATTQMQSTDARKSFPCFDEPAMKATFNITLIHPNNLTALSNMPPKGSSTPLAEDPNWSVTEFETTPVMSTYLLAYIVSEFQSVNETAQNGVLIRIWARPNAIAEGHGMYALNVTGPILNFFANHYNTSYPLPKSDQIALPDFNAGAMENWGLVTYRENALLFDPQSSSISNKERVVTVIAHELAHQWFGNLVTLAWWNDLWLNEGFASYVEYLGADHAEPTWNLKDLIVPGDVYRVMAVDALASSHPLTTPAEEVNTPAQISEMFDSISYSKGASVIRMLSNFLTEDLFKEGLASYLHAFAYQNTTYLDLWEHLQKAVDAQTSIRLPDTVRAIMDRWTLQMGFPVITVDTKTGNISQKHFLLDSESNVTRSSAFDYLWIVPISSIKNGVMQDHYWLRDVSQAQNDLFKTASDDWVLLNVNVTGYFQVNYDEDNWRMIQHQLQTNLSVIPVINRAQVIYDSFNLATAHMVPVTLALDNTLFLNGEKEYMPWQAALSSLSYFSLMFDRSEVYGPMKKYLRKQVEPLFQHFETLTKNWTERPENLMDQYSEINAISTACSNGLPQCENLAKTLFDQWMSDPENNPIHPNLRSTIYCNAIAQGGQDQWDFAWGQLQQAQLVNEADKLRSALACSNEVWLLNRYLGYTLNPDLIRKQDATSTINSIASNVIGQPLAWDFVQSNWKKLFQDYGGGSFSFSNLIQGVTRRFSSEFELQQLEQFKKNNMDVGFGSGTRALEQALEKTKANIKWVKENKEVVLNWFIEHS
Inhibitor
Name:
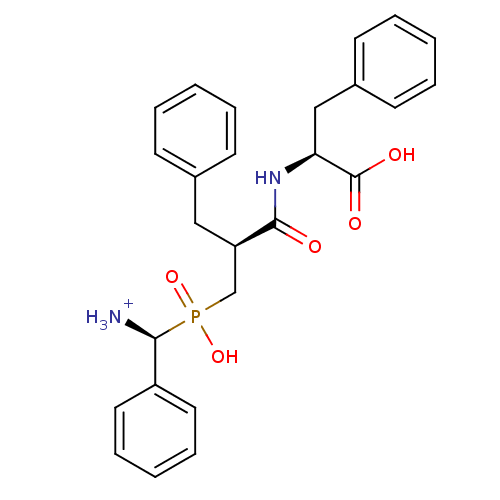
BDBM50087111
Synonyms:
(2S)-2-[(2S)-3-{[(S)-azaniumyl(phenyl)methyl](hydroxy)phosphoryl}-2-benzylpropanamido]-3-phenylpropanoic acid | C-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl-propyl]-hydroxy-phosphinoyl}-C-phenyl-methyl-ammonium
Type:
Small organic molecule
Emp. Form.:
C26H30N2O5P
Mol. Mass.:
481.5
SMILES:
[NH3+][C@H](c1ccccc1)P(O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(O)=O