Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50370258
Substrate
n/a
Meas. Tech.
ChEMBL_51929 (CHEMBL663577)
IC50
61000±n/a nM
Citation
 Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem 46:3778-81 (2003) [PubMed] Article
Wu, YJ; Davis, CD; Dworetzky, S; Fitzpatrick, WC; Harden, D; He, H; Knox, RJ; Newton, AE; Philip, T; Polson, C; Sivarao, DV; Sun, LQ; Tertyshnikova, S; Weaver, D; Yeola, S; Zoeckler, M; Sinz, MW Fluorine substitution can block CYP3A4 metabolism-dependent inhibition: identification of (S)-N-[1-(4-fluoro-3- morpholin-4-ylphenyl)ethyl]-3- (4-fluorophenyl)acrylamide as an orally bioavailable KCNQ2 opener devoid of CYP3A4 metabolism-dependent inhibition. J Med Chem 46:3778-81 (2003) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
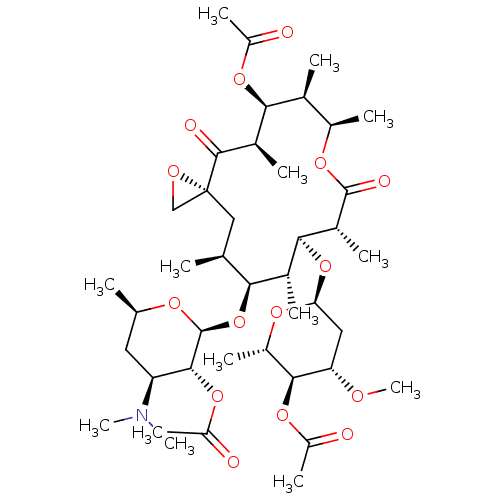
Inhibitor
Name:
BDBM50370258
Synonyms:
TROLEANDOMYCIN | Triacetyloleandomycin
Type:
Small organic molecule
Emp. Form.:
C41H67NO15
Mol. Mass.:
813.9684
SMILES:
CO[C@H]1C[C@H](O[C@H]2[C@H](C)[C@@H](O[C@@H]3O[C@H](C)C[C@@H]([C@H]3OC(C)=O)N(C)C)[C@@H](C)C[C@@]3(CO3)C(=O)[C@H](C)[C@@H](OC(C)=O)[C@@H](C)[C@@H](C)OC(=O)[C@@H]2C)O[C@@H](C)[C@@H]1OC(C)=O