Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Mu-type opioid receptor
Ligand
BDBM50346948
Substrate
n/a
Meas. Tech.
ChEMBL_753181 (CHEMBL1799274)
Ki
1.1±n/a nM
Citation
 Majumdar, S; Burgman, M; Haselton, N; Grinnell, S; Ocampo, J; Pasternak, AR; Pasternak, GW Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett 21:4001-4 (2011) [PubMed] Article
Majumdar, S; Burgman, M; Haselton, N; Grinnell, S; Ocampo, J; Pasternak, AR; Pasternak, GW Generation of novel radiolabeled opiates through site-selective iodination. Bioorg Med Chem Lett 21:4001-4 (2011) [PubMed] Article More Info.:
Target
Name:
Mu-type opioid receptor
Synonyms:
M-OR-1 | MOP | MOR-1 | MOR1 | MUOR1 | Mu Opioid Receptor | Mu opiate receptor | OPIATE Mu | OPRM1 | OPRM_HUMAN | hMOP | mu-type opioid receptor isoform MOR-1
Type:
G Protein-Coupled Receptor (GPCR)
Mol. Mass.:
44789.51
Organism:
Homo sapiens (Human)
Description:
P35372
Residue:
400
Sequence:
MDSSAAPTNASNCTDALAYSSCSPAPSPGSWVNLSHLDGNLSDPCGPNRTDLGGRDSLCPPTGSPSMITAITIMALYSIVCVVGLFGNFLVMYVIVRYTKMKTATNIYIFNLALADALATSTLPFQSVNYLMGTWPFGTILCKIVISIDYYNMFTSIFTLCTMSVDRYIAVCHPVKALDFRTPRNAKIINVCNWILSSAIGLPVMFMATTKYRQGSIDCTLTFSHPTWYWENLLKICVFIFAFIMPVLIITVCYGLMILRLKSVRMLSGSKEKDRNLRRITRMVLVVVAVFIVCWTPIHIYVIIKALVTIPETTFQTVSWHFCIALGYTNSCLNPVLYAFLDENFKRCFREFCIPTSSNIEQQNSTRIRQNTRDHPSTANTVDRTNHQLENLEAETAPLP
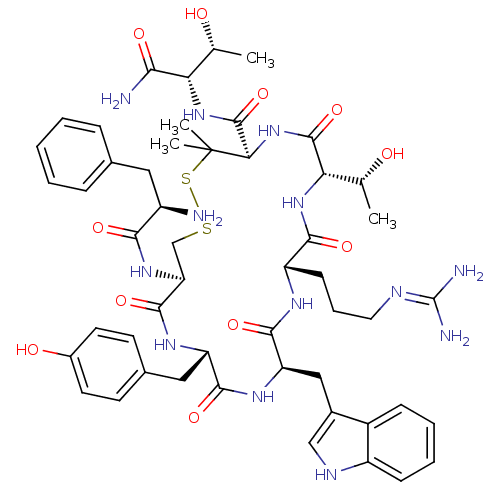
Inhibitor
Name:
BDBM50346948
Synonyms:
CHEMBL1795717 | CTAP-NH2
Type:
Small organic molecule
Emp. Form.:
C51H69N13O11S2
Mol. Mass.:
1104.304
SMILES:
C[C@@H](O)[C@H](NC(=O)[C@H]1NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC1(C)C)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r,wU:52.62,62.66,7.6,1.1,71.77,26.26,wD:3.3,11.75,15.15,40.42,(15.53,-44.79,;14.2,-44.02,;12.86,-44.79,;14.2,-42.47,;12.86,-41.71,;11.53,-42.47,;11.53,-44.02,;10.19,-41.71,;8.86,-42.47,;7.52,-41.71,;7.52,-40.16,;6.19,-42.47,;4.85,-41.71,;3.52,-42.47,;3.52,-44.02,;2.18,-41.71,;.85,-42.47,;-.49,-41.71,;-1.82,-42.47,;-3.16,-41.71,;-4.49,-42.47,;-5.82,-41.71,;-4.49,-44.02,;2.18,-40.16,;.85,-39.4,;-.49,-40.16,;.85,-37.85,;-.49,-37.09,;-1.82,-37.85,;-1.98,-39.4,;-3.49,-39.72,;-4.26,-38.38,;-5.76,-38.06,;-6.24,-36.61,;-5.2,-35.46,;-3.7,-35.78,;-3.23,-37.24,;2.18,-37.09,;2.18,-35.54,;.85,-34.77,;3.52,-34.77,;3.52,-33.23,;2.18,-32.46,;2.19,-30.92,;.85,-30.15,;-.48,-30.92,;-1.81,-30.15,;-.47,-32.47,;.86,-33.23,;4.85,-35.54,;6.19,-34.77,;6.19,-33.23,;7.52,-35.54,;7.52,-37.09,;8.86,-37.85,;8.86,-39.4,;10.19,-40.16,;10.95,-38.82,;11.66,-40.17,;8.86,-34.77,;10.19,-35.54,;10.19,-37.09,;11.53,-34.77,;12.86,-35.54,;11.53,-33.23,;12.86,-32.46,;14.19,-33.24,;15.52,-32.47,;15.52,-30.93,;14.18,-30.16,;12.86,-30.93,;6.19,-44.02,;7.52,-44.79,;4.85,-44.79,;15.53,-41.71,;16.87,-42.47,;15.53,-40.16,)|