Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Prostaglandin G/H synthase 2
Ligand
BDBM17636
Substrate
n/a
Meas. Tech.
ChEMBL_823530 (CHEMBL2045973)
IC50
16±n/a nM
Citation
 Adeniji, AO; Twenter, BM; Byrns, MC; Jin, Y; Chen, M; Winkler, JD; Penning, TM Development of potent and selective inhibitors of aldo-keto reductase 1C3 (type 5 17ß-hydroxysteroid dehydrogenase) based on N-phenyl-aminobenzoates and their structure-activity relationships. J Med Chem 55:2311-23 (2012) [PubMed] Article
Adeniji, AO; Twenter, BM; Byrns, MC; Jin, Y; Chen, M; Winkler, JD; Penning, TM Development of potent and selective inhibitors of aldo-keto reductase 1C3 (type 5 17ß-hydroxysteroid dehydrogenase) based on N-phenyl-aminobenzoates and their structure-activity relationships. J Med Chem 55:2311-23 (2012) [PubMed] Article More Info.:
Target
Name:
Prostaglandin G/H synthase 2
Synonyms:
COX2 | Cyclooxygenase | Cyclooxygenase 2 (COX-2) | Cyclooxygenase-2 | Cyclooxygenase-2 (COX-2 AA) | Cyclooxygenase-2 (COX-2 AEA) | Cyclooxygenase-2 (COX-2) | PGH synthase 2 | PGH2_HUMAN | PGHS-2 | PHS II | PTGS2 | Prostaglandin E synthase/G/H synthase 2 | Prostaglandin H2 synthase 2 | Prostaglandin-endoperoxide synthase 2
Type:
Enzyme
Mol. Mass.:
69003.89
Organism:
Homo sapiens (Human)
Description:
Recombinant Cox-2 provided by Cayman (Cayman Chemical Co.,Ann Arbor, MI).
Residue:
604
Sequence:
MLARALLLCAVLALSHTANPCCSHPCQNRGVCMSVGFDQYKCDCTRTGFYGENCSTPEFLTRIKLFLKPTPNTVHYILTHFKGFWNVVNNIPFLRNAIMSYVLTSRSHLIDSPPTYNADYGYKSWEAFSNLSYYTRALPPVPDDCPTPLGVKGKKQLPDSNEIVEKLLLRRKFIPDPQGSNMMFAFFAQHFTHQFFKTDHKRGPAFTNGLGHGVDLNHIYGETLARQRKLRLFKDGKMKYQIIDGEMYPPTVKDTQAEMIYPPQVPEHLRFAVGQEVFGLVPGLMMYATIWLREHNRVCDVLKQEHPEWGDEQLFQTSRLILIGETIKIVIEDYVQHLSGYHFKLKFDPELLFNKQFQYQNRIAAEFNTLYHWHPLLPDTFQIHDQKYNYQQFIYNNSILLEHGITQFVESFTRQIAGRVAGGRNVPPAVQKVSQASIDQSRQMKYQSFNEYRKRFMLKPYESFEELTGEKEMSAELEALYGDIDAVELYPALLVEKPRPDAIFGETMVEVGAPFSLKGLMGNVICSPAYWKPSTFGGEVGFQIINTASIQSLICNNVKGCPFTSFSVPDPELIKTVTINASSSRSGLDDINPTVLLKERSTEL
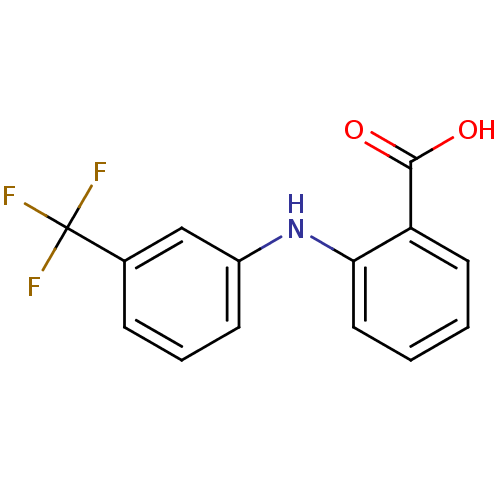
Inhibitor
Name:
BDBM17636
Synonyms:
2-{[3-(trifluoromethyl)phenyl]amino}benzoic acid | Arlef | CHEMBL23588 | Flufenamic acid | Nichisedan | US20240002326, Compound Flufenamic acid | US9271961, Flufenamic Acid
Type:
Small organic molecule
Emp. Form.:
C14H10F3NO2
Mol. Mass.:
281.2299
SMILES:
OC(=O)c1ccccc1Nc1cccc(c1)C(F)(F)F