Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
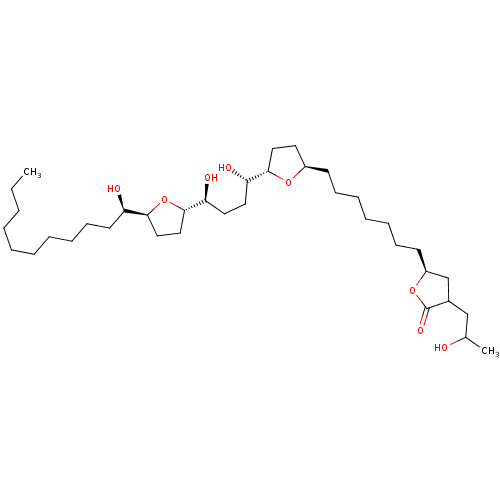
Ligand
BDBM50403906
Substrate
n/a
Meas. Tech.
ChEMBL_141738 (CHEMBL749250)
IC50
2.5±n/a nM
Citation
 Tormo, JR; Estornell, E; Gallardo, T; González, MC; Cavé, A; Granell, S; Cortes, D; Zafra-Polo, MC Gamma-lactone-Functionalized antitumoral acetogenins are the most potent inhibitors of mitochondrial complex I. Bioorg Med Chem Lett 11:681-4 (2001) [PubMed] Article
Tormo, JR; Estornell, E; Gallardo, T; González, MC; Cavé, A; Granell, S; Cortes, D; Zafra-Polo, MC Gamma-lactone-Functionalized antitumoral acetogenins are the most potent inhibitors of mitochondrial complex I. Bioorg Med Chem Lett 11:681-4 (2001) [PubMed] Article More Info.:
Target
Name:
Acyl carrier protein,/NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
Synonyms:
Mitochondrial complex I; NADH oxidoreductase
Type:
n/a
Mol. Mass.:
n/a
Description:
ASSAY_ID of EBI is 141738
Components:
This complex has 2 components.
Component 1
Name:
NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
Synonyms:
CI-51kD | Complex I-51kD | NADH dehydrogenase flavoprotein 1 | NADH-ubiquinone oxidoreductase 51 kDa subunit | NDUFV1 | NDUV1_BOVIN | UQOR1
Type:
PROTEIN
Mol. Mass.:
50659.85
Organism:
Bos taurus
Description:
EBI_100771
Residue:
464
Sequence:
MLAARRLLGGSLPARVSVRFSGDTTAPKKTSFGSLKDEDRIFTNLYGRHDWRLKGAQSRGDWYKTKEILLKGPDWILGEVKTSGLRGRGGAGFPTGLKWSFMNKPSDGRPKYLVVNADEGEPGTCKDREIIRHDPHKLVEGCLVGGRAMGARAAYIYIRGEFYNEASNLQVAIREAYEAGLIGKNACGSGYDFDVFVVRGAGAYICGEETALIESIEGKQGKPRLKPPFPADVGVFGCPTTVANVETVAVSPTICRRGGAWFASFGRERNSGTKLFNISGHVNNPCTVEEEMSVPLKELIEKHAGGVTGGWDNLLAVIPGGSSTPLIPKSVCETVLMDFDALIQAQTGLGTAAVIVMDRSTDIVKAIARLIEFYKHESCGQCTPCREGVDWMNKVMARFVRGDARPAEIDSLWEISKQIEGHTICALGDGAAWPVQGLIRHFRPELEERMQQFAQQHQARQAAF
Component 2
Name:
Acyl carrier protein, mitochondrial
Synonyms:
ACP | ACPM_BOVIN | CI-SDAP | NADH-ubiquinone oxidoreductase 9.6 kDa subunit | NDUFAB1
Type:
PROTEIN
Mol. Mass.:
17397.64
Organism:
Bos taurus
Description:
ChEMBL_469770
Residue:
156
Sequence:
MAVRVLCACVRRLPTAFAPLPRLPTLAAARPLSTTLFAAETRTRPGAPLPALVLAQVPGRVTQLCRQYSDAPPLTLEGIKDRVLYVLKLYDKIDPEKLSVNSHFMKDLGLDSLDQVEIIMAMEDEFGFEIPDIDAEKLMCPQEIVDYIADKKDVYE