Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Acetylcholinesterase
Ligand
BDBM50333779
Substrate
n/a
Meas. Tech.
ChEMBL_1350415 (CHEMBL3268869)
Kd
21480±n/a nM
Citation
 Karade, HN; Valiveti, AK; Acharya, J; Kaushik, MP Synthesis and in vitro evaluation of bis-quaternary 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide derivatives as reactivators against sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem 22:2684-91 (2014) [PubMed] Article
Karade, HN; Valiveti, AK; Acharya, J; Kaushik, MP Synthesis and in vitro evaluation of bis-quaternary 2-(hydroxyimino)-N-(pyridin-3-yl)acetamide derivatives as reactivators against sarin and VX inhibited human acetylcholinesterase (hAChE). Bioorg Med Chem 22:2684-91 (2014) [PubMed] Article More Info.:
Target
Name:
Acetylcholinesterase
Synonyms:
ACES_HUMAN | ACHE | Acetylcholinesterase (AChE) | Acetylcholinesterase (human AChE)
Type:
Enzyme
Mol. Mass.:
67792.70
Organism:
Homo sapiens (Human)
Description:
P22303
Residue:
614
Sequence:
MRPPQCLLHTPSLASPLLLLLLWLLGGGVGAEGREDAELLVTVRGGRLRGIRLKTPGGPVSAFLGIPFAEPPMGPRRFLPPEPKQPWSGVVDATTFQSVCYQYVDTLYPGFEGTEMWNPNRELSEDCLYLNVWTPYPRPTSPTPVLVWIYGGGFYSGASSLDVYDGRFLVQAERTVLVSMNYRVGAFGFLALPGSREAPGNVGLLDQRLALQWVQENVAAFGGDPTSVTLFGESAGAASVGMHLLSPPSRGLFHRAVLQSGAPNGPWATVGMGEARRRATQLAHLVGCPPGGTGGNDTELVACLRTRPAQVLVNHEWHVLPQESVFRFSFVPVVDGDFLSDTPEALINAGDFHGLQVLVGVVKDEGSYFLVYGAPGFSKDNESLISRAEFLAGVRVGVPQVSDLAAEAVVLHYTDWLHPEDPARLREALSDVVGDHNVVCPVAQLAGRLAAQGARVYAYVFEHRASTLSWPLWMGVPHGYEIEFIFGIPLDPSRNYTAEEKIFAQRLMRYWANFARTGDPNEPRDPKAPQWPPYTAGAQQYVSLDLRPLEVRRGLRAQACAFWNRFLPKLLSATDTLDEAERQWKAEFHRWSSYMVHWKNQFDHYSKQDRCSDL
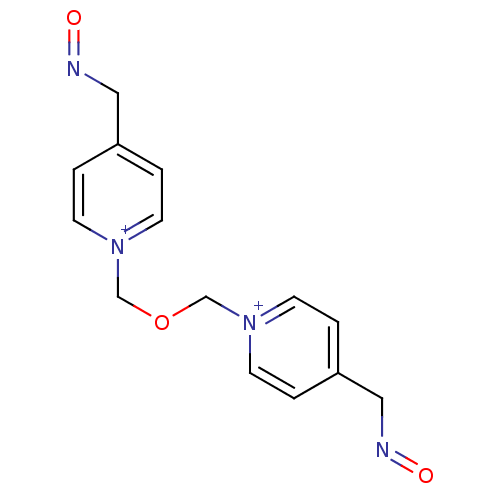
Inhibitor
Name:
BDBM50333779
Synonyms:
1,3-Bis(4-(Hydroxyimino-methyl)-pyridinium)-2-oxapropane; dichloride(Toxogonin) | 1,3-bis(4-hydroxyiminomethylpyridinium)-2-oxa-propane dichloride | 1,3-bis(4-hydroxyiminomethylpyridinium)-2-oxapropane dichloride | 4-[(E)-(hydroxyimino)methyl]-1-[({4-[(E)-(hydroxyimino)methyl]pyridinium-1-yl}methoxy)methyl]pyridinium dichloride | 4-[(E)-(hydroxyimino)methyl]-1-[({4-[(E)-(hydroxyimino)methyl]pyridinium-1-yl}methoxy)methyl]pyridinium dichloride(toxogonin) | 4-[(hydroxyimino)methyl]-1-[({4-[(hydroxyimino)methyl]pyridinium-1-yl}methoxy)methyl]pyridinium dichloride | CHEMBL291233 | Obidoxime chloride | Toxogonin | obidoxime
Type:
Small organic molecule
Emp. Form.:
C14H16N4O3
Mol. Mass.:
288.3007
SMILES:
O=NCc1cc[n+](COC[n+]2ccc(CN=O)cc2)cc1