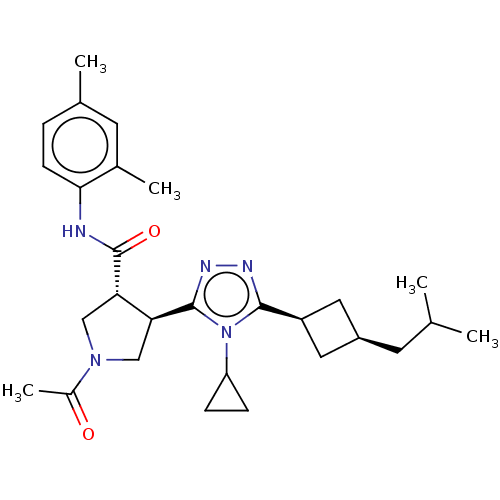
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Peroxisome proliferator-activated receptor alpha
Ligand
BDBM50153594
Substrate
n/a
Meas. Tech.
ChEMBL_1561278 (CHEMBL3778098)
EC50
>20000±n/a nM
Citation
 Hirata, K; Kotoku, M; Seki, N; Maeba, T; Maeda, K; Hirashima, S; Sakai, T; Obika, S; Hori, A; Hase, Y; Yamaguchi, T; Katsuda, Y; Hata, T; Miyagawa, N; Arita, K; Nomura, Y; Asahina, K; Aratsu, Y; Kamada, M; Adachi, T; Noguchi, M; Doi, S; Crowe, P; Bradley, E; Steensma, R; Tao, H; Fenn, M; Babine, R; Li, X; Thacher, S; Hashimoto, H; Shiozaki, M SAR Exploration Guided by LE and Fsp(3): Discovery of a Selective and Orally Efficacious ROR� Inhibitor. ACS Med Chem Lett 7:23-7 (2016) [PubMed] Article
Hirata, K; Kotoku, M; Seki, N; Maeba, T; Maeda, K; Hirashima, S; Sakai, T; Obika, S; Hori, A; Hase, Y; Yamaguchi, T; Katsuda, Y; Hata, T; Miyagawa, N; Arita, K; Nomura, Y; Asahina, K; Aratsu, Y; Kamada, M; Adachi, T; Noguchi, M; Doi, S; Crowe, P; Bradley, E; Steensma, R; Tao, H; Fenn, M; Babine, R; Li, X; Thacher, S; Hashimoto, H; Shiozaki, M SAR Exploration Guided by LE and Fsp(3): Discovery of a Selective and Orally Efficacious ROR� Inhibitor. ACS Med Chem Lett 7:23-7 (2016) [PubMed] Article More Info.:
Target
Name:
Peroxisome proliferator-activated receptor alpha
Synonyms:
NR1C1 | Nuclear receptor subfamily 1 group C member 1 | PPAR | PPAR alpha/gamma | PPAR-alpha | PPARA | PPARA_HUMAN | Peroxisome Proliferator-Activated Receptor alpha | Peroxisome proliferator-activated receptor | Peroxisome proliferator-activated receptor alpha (PPAR alpha)
Type:
Enzyme
Mol. Mass.:
52222.08
Organism:
Homo sapiens (Human)
Description:
Q07869
Residue:
468
Sequence:
MVDTESPLCPLSPLEAGDLESPLSEEFLQEMGNIQEISQSIGEDSSGSFGFTEYQYLGSCPGSDGSVITDTLSPASSPSSVTYPVVPGSVDESPSGALNIECRICGDKASGYHYGVHACEGCKGFFRRTIRLKLVYDKCDRSCKIQKKNRNKCQYCRFHKCLSVGMSHNAIRFGRMPRSEKAKLKAEILTCEHDIEDSETADLKSLAKRIYEAYLKNFNMNKVKARVILSGKASNNPPFVIHDMETLCMAEKTLVAKLVANGIQNKEAEVRIFHCCQCTSVETVTELTEFAKAIPGFANLDLNDQVTLLKYGVYEAIFAMLSSVMNKDGMLVAYGNGFITREFLKSLRKPFCDIMEPKFDFAMKFNALELDDSDISLFVAAIICCGDRPGLLNVGHIEKMQEGIVHVLRLHLQSNHPDDIFLFPKLLQKMADLRQLVTEHAQLVQIIKKTESDAALHPLLQEIYRDMY
Inhibitor
Name:
BDBM50153594
Synonyms:
CHEMBL3774855
Type:
Small organic molecule
Emp. Form.:
C28H39N5O2
Mol. Mass.:
477.6416
SMILES:
CC(C)C[C@H]1C[C@H](C1)c1nnc([C@H]2CN(C[C@@H]2C(=O)Nc2ccc(C)cc2C)C(C)=O)n1C1CC1 |r,wU:4.3,6.8,12.12,wD:16.18,(2.36,7.33,;2.68,6.14,;1.81,5.27,;4.17,5.74,;4.56,4.26,;5.9,3.48,;5.11,2.21,;3.79,2.92,;5.62,.75,;7.1,.32,;7.14,-1.22,;5.7,-1.71,;5.38,-3.22,;6.42,-4.36,;5.65,-5.7,;4.14,-5.38,;4,-3.86,;2.66,-3.08,;2.67,-1.85,;1.33,-3.85,;-.01,-3.08,;0,-1.54,;-1.33,-.76,;-2.67,-1.53,;-3.73,-.91,;-2.67,-3.07,;-1.34,-3.84,;-1.35,-5.07,;6.27,-7.1,;5.55,-8.1,;7.5,-7.23,;4.75,-.52,;3.21,-.57,;1.93,-1.26,;2.03,.28,)|