Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Pancreatic triacylglycerol lipase
Ligand
BDBM50207385
Substrate
n/a
Meas. Tech.
ChEBML_1631109
IC50
17900±n/a nM
Citation
 Sridhar, SN; Ginson, G; Venkataramana Reddy, PO; Tantak, MP; Kumar, D; Paul, AT Synthesis, evaluation and molecular modelling studies of 2-(carbazol-3-yl)-2-oxoacetamide analogues as a new class of potential pancreatic lipase inhibitors. Bioorg Med Chem 25:609-620 (2017) [PubMed] Article
Sridhar, SN; Ginson, G; Venkataramana Reddy, PO; Tantak, MP; Kumar, D; Paul, AT Synthesis, evaluation and molecular modelling studies of 2-(carbazol-3-yl)-2-oxoacetamide analogues as a new class of potential pancreatic lipase inhibitors. Bioorg Med Chem 25:609-620 (2017) [PubMed] Article More Info.:
Target
Name:
Pancreatic triacylglycerol lipase
Synonyms:
LIPP_HUMAN | PL | PNLIP | Pancreatic lipase
Type:
PROTEIN
Mol. Mass.:
51157.75
Organism:
Homo sapiens (Human)
Description:
ChEMBL_527250
Residue:
465
Sequence:
MLPLWTLSLLLGAVAGKEVCYERLGCFSDDSPWSGITERPLHILPWSPKDVNTRFLLYTNENPNNFQEVAADSSSISGSNFKTNRKTRFIIHGFIDKGEENWLANVCKNLFKVESVNCICVDWKGGSRTGYTQASQNIRIVGAEVAYFVEFLQSAFGYSPSNVHVIGHSLGAHAAGEAGRRTNGTIGRITGLDPAEPCFQGTPELVRLDPSDAKFVDVIHTDGAPIVPNLGFGMSQVVGHLDFFPNGGVEMPGCKKNILSQIVDIDGIWEGTRDFAACNHLRSYKYYTDSIVNPDGFAGFPCASYNVFTANKCFPCPSGGCPQMGHYADRYPGKTNDVGQKFYLDTGDASNFARWRYKVSVTLSGKKVTGHILVSLFGNKGNSKQYEIFKGTLKPDSTHSNEFDSDVDVGDLQMVKFIWYNNVINPTLPRVGASKIIVETNVGKQFNFCSPETVREEVLLTLTPC
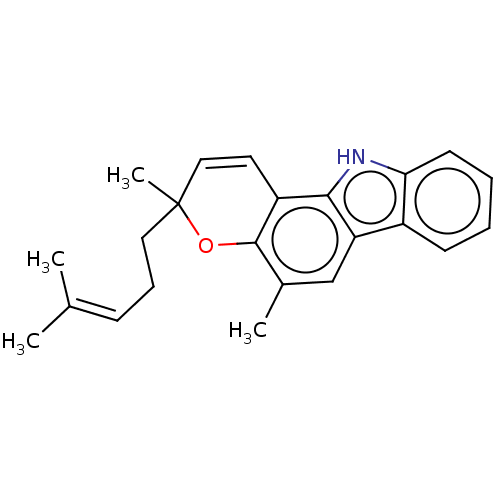
Inhibitor
Name:
BDBM50207385
Synonyms:
CHEBI:6646 | Mahanimbine | Rel-(+)-Mahanimbine
Type:
Small organic molecule
Emp. Form.:
C23H25NO
Mol. Mass.:
331.4507
SMILES:
CC(C)=CCCC1(C)Oc2c(C)cc3c([nH]c4ccccc34)c2C=C1 |c:26,(11.36,-24.12,;9.81,-24.12,;9.04,-25.45,;9.04,-22.78,;7.5,-22.77,;6.74,-21.43,;5.19,-21.43,;5.96,-20.08,;3.86,-20.66,;2.52,-21.44,;1.19,-20.67,;1.19,-19.13,;-.14,-21.44,;-.14,-22.98,;1.2,-23.74,;.88,-25.25,;-.65,-25.41,;-1.57,-26.66,;-3.11,-26.49,;-3.73,-25.09,;-2.81,-23.84,;-1.28,-24,;2.52,-22.97,;3.86,-23.73,;5.18,-22.96,)|