Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Sucrase-isomaltase, intestinal
Ligand
BDBM23406
Substrate
n/a
Meas. Tech.
ChEMBL_1645848 (CHEMBL3994904)
IC50
20200±n/a nM
Citation
More Info.:
Target
Name:
Sucrase-isomaltase, intestinal
Synonyms:
SUIS_RAT | Si | Sucrase-isomaltase | alpha-Glucosidase (α-Glucosidase)
Type:
Enzyme
Mol. Mass.:
210329.04
Organism:
Rattus norvegicus (Rat)
Description:
P23739
Residue:
1841
Sequence:
MAKKKFSALEISLIVLFIIVTAIAIALVTVLATKVPAVEEIKSPTPTSNSTPTSTPTSTSTPTSTSTPSPGKCPPEQGEPINERINCIPEQHPTKAICEERGCCWRPWNNTVIPWCFFADNHGYNAESITNENAGLKATLNRIPSPTLFGEDIKSVILTTQTQTGNRFRFKITDPNNKRYEVPHQFVKEETGIPAADTLYDVQVSENPFSIKVIRKSNNKVLCDTSVGPLLYSNQYLQISTRLPSEYIYGFGGHIHKRFRHDLYWKTWPIFTRDEIPGDNNHNLYGHQTFFMGIGDTSGKSYGVFLMNSNAMEVFIQPTPIITYRVTGGILDFYIFLGDTPEQVVQQYQEVHWRPAMPAYWNLGFQLSRWNYGSLDTVSEVVRRNREAGIPYDAQVTDIDYMEDHKEFTYDRVKFNGLPEFAQDLHNHGKYIIILDPAISINKRANGAEYQTYVRGNEKNVWVNESDGTTPLIGEVWPGLTVYPDFTNPQTIEWWANECNLFHQQVEYDGLWIDMNEVSSFIQGSLNLKGVLLIVLNYPPFTPGILDKVMYSKTLCMDAVQHWGKQYDVHSLYGYSMAIATEQAVERVFPNKRSFILTRSTFGGSGRHANHWLGDNTASWEQMEWSITGMLEFGIFGMPLVGATSCGFLADTTEELCRRWMQLGAFYPFSRNHNAEGYMEQDPAYFGQDSSRHYLTIRYTLLPFLYTLFYRAHMFGETVARPFLYEFYDDTNSWIEDTQFLWGPALLITPVLRPGVENVSAYIPNATWYDYETGIKRPWRKERINMYLPGDKIGLHLRGGYIIPTQEPDVTTTASRKNPLGLIVALDDNQAAKGELFWDDGESKDSIEKKMYILYTFSVSNNELVLNCTHSSYAEGTSLAFKTIKVLGLREDVRSITVGENDQQMATHTNFTFDSANKILSITALNFNLAGSFIVRWCRTFSDNEKFTCYPDVGTATEGTCTQRGCLWQPVSGLSNVPPYYFPPENNPYTLTSIQPLPTGITAELQLNPPNARIKLPSNPISTLRVGVKYHPNDMLQFKIYDAQHKRYEVPVPLNIPDTPTSSNERLYDVEIKENPFGIQVRRRSSGKLIWDSRLPGFGFNDQFIQISTRLPSNYLYGFGEVEHTAFKRDLNWHTWGMFTRDQPPGYKLNSYGFHPYYMALENEGNAHGVLLLNSNGMDVTFQPTPALTYRTIGGILDFYMFLGPTPEIATRQYHEVIGFPVMPPYWALGFQLCRYGYRNTSEIEQLYNDMVAANIPYDVQYTDINYMERQLDFTIGERFKTLPEFVDRIRKDGMKYIVILAPAISGNETQPYPAFERGIQKDVFVKWPNTNDICWPKVWPDLPNVTIDETITEDEAVNASRAHVAFPDFFRNSTLEWWAREIYDFYNEKMKFDGLWIDMNEPSSFGIQMGGKVLNECRRMMTLNYPPVFSPELRVKEGEGASISEAMCMETEHILIDGSSVLQYDVHNLYGWSQVKPTLDALQNTTGLRGIVISRSTYPTTGRWGGHWLGDNYTTWDNLEKSLIGMLELNLFGIPYIGADICGVFHDSGYPSLYFVGIQVGAFYPYPRESPTINFTRSQDPVSWMKLLLQMSKKVLEIRYTLLPYFYTQMHEAHAHGGTVIRPLMHEFFDDKETWEIYKQFLWGPAFMVTPVVEPFRTSVTGYVPKARWFDYHTGADIKLKGILHTFSAPFDTINLHVRGGYILPCQEPARNTHLSRQNYMKLIVAADDNQMAQGTLFGDDGESIDTYERGQYTSIQFNLNQTTLTSTVLANGYKNKQEMRLGSIHIWGKGTLRISNANLVYGGRKHQPPFTQEEAKETLIFDLKNMNVTLDEPIQITWS
Inhibitor
Name:
BDBM23406
Synonyms:
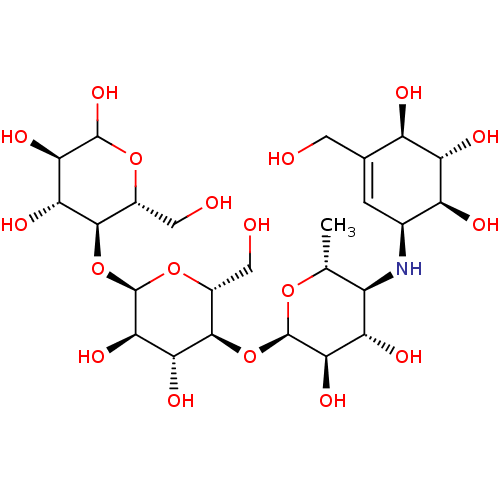
(3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5-{[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl-5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohex-2-en-1-yl]amino}oxan-2-yl]oxy}-3,4-dihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-6-(hydroxymethyl)oxane-2,3,4-triol | Acarbose | US11292789, Acarbose
Type:
Carbohydrate
Emp. Form.:
C25H43NO18
Mol. Mass.:
645.6048
SMILES:
C[C@H]1O[C@H](O[C@@H]2[C@@H](CO)O[C@H](O[C@@H]3[C@@H](CO)OC(O)[C@H](O)[C@H]3O)[C@H](O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1N[C@H]1C=C(CO)[C@@H](O)[C@H](O)[C@H]1O |t:37|