Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone-lysine N-methyltransferase, H3 lysine-79 specific
Ligand
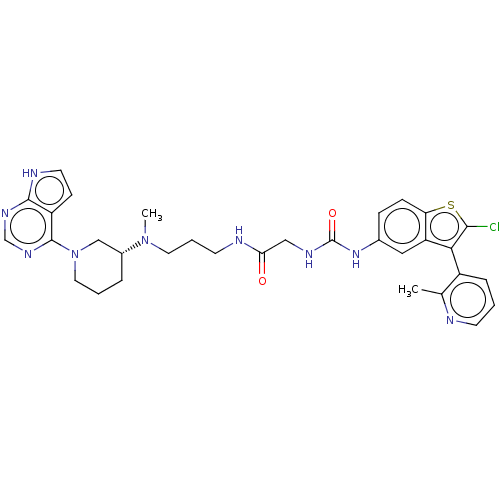
BDBM50235302
Substrate
n/a
Meas. Tech.
ChEMBL_1655315 (CHEMBL4004681)
IC50
17±n/a nM
Citation
 M÷bitz, H; Machauer, R; Holzer, P; Vaupel, A; Stauffer, F; Ragot, C; Caravatti, G; Scheufler, C; Fernandez, C; Hommel, U; Tiedt, R; Beyer, KS; Chen, C; Zhu, H; Gaul, C Discovery of Potent, Selective, and Structurally Novel Dot1L Inhibitors by a Fragment Linking Approach. ACS Med Chem Lett 8:338-343 (2017) [PubMed] Article
M÷bitz, H; Machauer, R; Holzer, P; Vaupel, A; Stauffer, F; Ragot, C; Caravatti, G; Scheufler, C; Fernandez, C; Hommel, U; Tiedt, R; Beyer, KS; Chen, C; Zhu, H; Gaul, C Discovery of Potent, Selective, and Structurally Novel Dot1L Inhibitors by a Fragment Linking Approach. ACS Med Chem Lett 8:338-343 (2017) [PubMed] Article More Info.:
Target
Name:
Histone-lysine N-methyltransferase, H3 lysine-79 specific
Synonyms:
2.1.1.43 | DOT1-like protein | DOT1-like protein (Dot1L) | DOT1L | DOT1L_HUMAN | H3-K79-HMTase | Histone H3-K79 methyltransferase | Histone H3-K79 methyltransferase (DOT1L) | Histone Methyltransferase DOT1L | Histone-lysine N-methyltransferase, H3 lysine-79 specific (DOT1L) | KIAA1814 | KMT4 | Lysine N-methyltransferase 4
Type:
Protein
Mol. Mass.:
184911.91
Organism:
Homo sapiens (Human)
Description:
Q8TEK3
Residue:
1537
Sequence:
MGEKLELRLKSPVGAEPAVYPWPLPVYDKHHDAAHEIIETIRWVCEEIPDLKLAMENYVLIDYDTKSFESMQRLCDKYNRAIDSIHQLWKGTTQPMKLNTRPSTGLLRHILQQVYNHSVTDPEKLNNYEPFSPEVYGETSFDLVAQMIDEIKMTDDDLFVDLGSGVGQVVLQVAAATNCKHHYGVEKADIPAKYAETMDREFRKWMKWYGKKHAEYTLERGDFLSEEWRERIANTSVIFVNNFAFGPEVDHQLKERFANMKEGGRIVSSKPFAPLNFRINSRNLSDIGTIMRVVELSPLKGSVSWTGKPVSYYLHTIDRTILENYFSSLKNPKLREEQEAARRRQQRESKSNAATPTKGPEGKVAGPADAPMDSGAEEEKAGAATVKKPSPSKARKKKLNKKGRKMAGRKRGRPKKMNTANPERKPKKNQTALDALHAQTVSQTAASSPQDAYRSPHSPFYQLPPSVQRHSPNPLLVAPTPPALQKLLESFKIQYLQFLAYTKTPQYKASLQELLGQEKEKNAQLLGAAQQLLSHCQAQKEEIRRLFQQKLDELGVKALTYNDLIQAQKEISAHNQQLREQSEQLEQDNRALRGQSLQLLKARCEELQLDWATLSLEKLLKEKQALKSQISEKQRHCLELQISIVELEKSQRQQELLQLKSCVPPDDALSLHLRGKGALGRELEPDASRLHLELDCTKFSLPHLSSMSPELSMNGQAAGYELCGVLSRPSSKQNTPQYLASPLDQEVVPCTPSHVGRPRLEKLSGLAAPDYTRLSPAKIVLRRHLSQDHTVPGRPAASELHSRAEHTKENGLPYQSPSVPGSMKLSPQDPRPLSPGALQLAGEKSSEKGLRERAYGSSGELITSLPISIPLSTVQPNKLPVSIPLASVVLPSRAERARSTPSPVLQPRDPSSTLEKQIGANAHGAGSRSLALAPAGFSYAGSVAISGALAGSPASLTPGAEPATLDESSSSGSLFATVGSRSSTPQHPLLLAQPRNSLPASPAHQLSSSPRLGGAAQGPLPEASKGDLPSDSGFSDPESEAKRRIVFTITTGAGSAKQSPSSKHSPLTASARGDCVPSHGQDSRRRGRRKRASAGTPSLSAGVSPKRRALPSVAGLFTQPSGSPLNLNSMVSNINQPLEITAISSPETSLKSSPVPYQDHDQPPVLKKERPLSQTNGAHYSPLTSDEEPGSEDEPSSARIERKIATISLESKSPPKTLENGGGLAGRKPAPAGEPVNSSKWKSTFSPISDIGLAKSADSPLQASSALSQNSLFTFRPALEEPSADAKLAAHPRKGFPGSLSGADGLSPGTNPANGCTFGGGLAADLSLHSFSDGASLPHKGPEAAGLSSPLSFPSQRGKEGSDANPFLSKRQLDGLAGLKGEGSRGKEAGEGGLPLCGPTDKTPLLSGKAAKARDREVDLKNGHNLFISAAAVPPGSLLSGPGLAPAASSAGGAASSAQTHRSFLGPFPPGPQFALGPMSLQANLGSVAGSSVLQSLFSSVPAAAGLVHVSSAATRLTNSHAMGSFSGVAGGTVGGN
Inhibitor
Name:
BDBM50235302
Synonyms:
CHEMBL4099771
Type:
Small organic molecule
Emp. Form.:
C32H36ClN9O2S
Mol. Mass.:
646.205
SMILES:
CN(CCCNC(=O)CNC(=O)Nc1ccc2sc(Cl)c(-c3cccnc3C)c2c1)[C@@H]1CCCN(C1)c1ncnc2[nH]ccc12 |r,wU:30.32,(40.26,-16.4,;40.26,-17.94,;41.59,-18.71,;42.93,-17.94,;44.26,-18.71,;45.59,-17.94,;46.93,-18.71,;46.93,-20.25,;48.26,-17.94,;49.59,-18.71,;50.93,-17.94,;50.93,-16.4,;52.26,-18.71,;53.6,-17.95,;53.59,-16.41,;54.92,-15.64,;56.26,-16.41,;57.73,-15.93,;58.64,-17.18,;60.18,-17.18,;57.73,-18.43,;58.21,-19.89,;57.17,-21.03,;57.65,-22.5,;59.16,-22.82,;60.19,-21.66,;59.71,-20.2,;60.74,-19.06,;56.26,-17.95,;54.93,-18.72,;38.93,-18.71,;37.59,-17.93,;36.26,-18.71,;36.26,-20.25,;37.59,-21.01,;38.93,-20.24,;37.59,-22.55,;38.93,-23.31,;38.93,-24.86,;37.6,-25.63,;36.26,-24.86,;34.8,-25.34,;33.89,-24.1,;34.79,-22.85,;36.26,-23.32,)|