Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Promotilin
Ligand
BDBM50127141
Substrate
n/a
Ki
1000±n/a nM
Comments
PDSP_6110
Citation
 Clark, MJ; Wright, T; Bertrand, PP; Bornstein, JC; Jenkinson, KM; Verlinden, M; Furness, JB Erythromycin derivatives ABT 229 and GM 611 act on motilin receptors in the rabbit duodenum. Clin Exp Pharmacol Physiol 26:242-5 (1999) [PubMed] Article
Clark, MJ; Wright, T; Bertrand, PP; Bornstein, JC; Jenkinson, KM; Verlinden, M; Furness, JB Erythromycin derivatives ABT 229 and GM 611 act on motilin receptors in the rabbit duodenum. Clin Exp Pharmacol Physiol 26:242-5 (1999) [PubMed] Article More Info.:
Target
Name:
Promotilin
Synonyms:
MLN | MOTI_RABIT | Motilin | Promotilin
Type:
Enzyme Catalytic Domain
Mol. Mass.:
14668.99
Organism:
RABBIT
Description:
Motilin 0 RABBIT::P27114
Residue:
133
Sequence:
MVSRKAVAALLLVHVTAMLASQTEAFVPIFTYSELQRMQERERNRGHKKSLSVQQRSDAAAAPRPAEPTLEEENGRMQLTAPVEIGMRMNSRQLEKYRAALEAAERAVHPDAPSRPCWPAGGESGWSGEPSPT
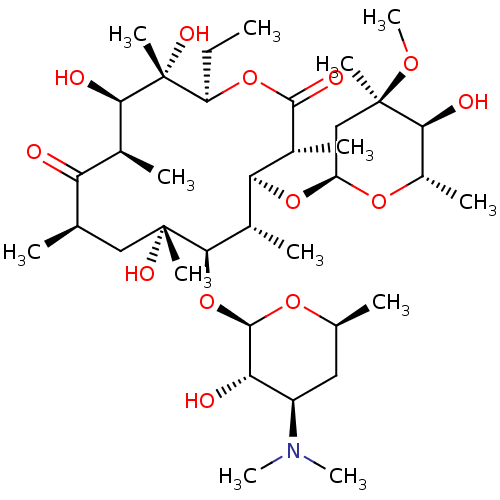
Inhibitor
Name:
BDBM50127141
Synonyms:
(3R,4S,5S,6R,7R,9R,11R,12R,13S,14R)-6-{[(2R,3S,4R,6S)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy}-14-ethyl-7,12,13-trihydroxy-4-{[(2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyloxan-2-yl]oxy}-3,5,7,9,11,13-hexamethyl-1-oxacyclotetradecane-2,10-dione | 11-(4-dimethylamino-3-hydroxy-6-methyltetrahydro-2H-2-pyranyloxy)-3-ethyl-4,5,10-trihydroxy-13-(5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-2-pyranyloxy)-4,6,8,10,12,14-hexamethyl-2-oxacyclotetradecane-1,7-dione | CHEMBL532 | Eryhtromycin A | erythromycin | erythromycin-A
Type:
Small organic molecule
Emp. Form.:
C37H67NO13
Mol. Mass.:
733.9268
SMILES:
CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@H]2O[C@@H](C)C[C@H]([C@@H]2O)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O |r|