Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
Ligand
BDBM278697
Substrate
n/a
Meas. Tech.
Inhibitory Activity Against IDH1R132H and IDH1R132C Enzymes
pH
7.5±n/a
Temperature
298.15±n/a K
IC50
11±n/a nM
Comments
extracted
Citation
 Saito, S; Itoh, M; Fujisawa, T; Saito, H; Kiyotsuka, Y; Watanabe, H; Matsunaga, H; Kagoshima, Y; Suzuki, T; Ogawara, Y; Kitabayashi, K Isoxazole derivative as mutant isocitrate dehydrogenase 1 inhibitor US Patent US10040791 Publication Date 8/7/2018
Saito, S; Itoh, M; Fujisawa, T; Saito, H; Kiyotsuka, Y; Watanabe, H; Matsunaga, H; Kagoshima, Y; Suzuki, T; Ogawara, Y; Kitabayashi, K Isoxazole derivative as mutant isocitrate dehydrogenase 1 inhibitor US Patent US10040791 Publication Date 8/7/2018 More Info.:
Target
Name:
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
Synonyms:
Cytosolic NADP-isocitrate dehydrogenase (IDH1)(R132H) | IDH1 | IDH1 R132H | IDH1(R132H) | IDHC_HUMAN | Isocitrate dehydrogenase (IDH1)(R132H) | Isocitrate dehydrogenase 1 mutant (R132H) | Isocitrate dehydrogenase [NADP] cytoplasmic (IDH)(R132H) | Isocitrate dehydrogenase [NADP] cytoplasmic (IDH1)(R132H) | Isocitrate dehydrogenase [NADP] cytoplasmic (R132H) | PICD
Type:
Protein
Mol. Mass.:
46641.74
Organism:
Homo sapiens (Human)
Description:
Human IDH1 R132H (SEQ ID No. 2 in patent). First three are removed. Google patent parsed wrong.
Residue:
414
Sequence:
MSKKISGGSVVEMQGDEMTRIIWELIKEKLIFPYVELDLHSYDLGIENRDATNDQVTKDAAEAIKKHNVGVKCATITPDEKRVEEFKLKQMWKSPNGTIRNILGGTVFREAIICKNIPRLVSGWVKPIIIGHHAYGDQYRATDFVVPGPGKVEITYTPSDGTQKVTYLVHNFEEGGGVAMGMYNQDKSIEDFAHSSFQMALSKGWPLYLSTKNTILKKYDGRFKDIFQEIYDKQYKSQFEAQKIWYEHRLIDDMVAQAMKSEGGFIWACKNYDGDVQSDSVAQGYGSLGMMTSVLVCPDGKTVEAEAAHGTVTRHYRMYQKGQETSTNPIASIFAWTRGLAHRAKLDNNKELAFFANALEEVSIETIEAGFMTKDLAACIKGLPNVQRSDYLNTFEFMDKLGENLKIKLAQAKL
Inhibitor
Name:
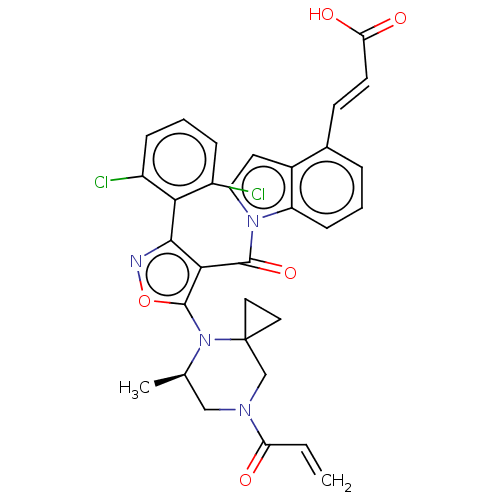
BDBM278697
Synonyms:
(2E)-3-[1-({5-[(5R)7-Acryloyl-5-methyl-4,7-diazaspiro[2.5]oct-4-yl]-3-(2,6-dichlorophenyl)-1,2-oxazol-4-yl}carbonyl)-1H-indol-4-yl]prop-2-enoic acid | US10040791, Example 159
Type:
Small organic molecule
Emp. Form.:
C31H26Cl2N4O5
Mol. Mass.:
605.468
SMILES:
C[C@@H]1CN(CC2(CC2)N1c1onc(c1C(=O)n1ccc2c(\C=C\C(O)=O)cccc12)-c1c(Cl)cccc1Cl)C(=O)C=C |r,wU:1.0,(-.54,-.23,;.55,.86,;2.03,1.26,;2.43,2.75,;1.34,3.83,;-.14,3.44,;-.14,4.98,;-1.48,4.21,;-.54,1.95,;-2.03,1.55,;-3.28,2.46,;-4.52,1.55,;-4.05,.09,;-2.51,.09,;-1.74,-1.25,;-2.51,-2.58,;-.2,-1.25,;.71,-0,;2.17,-.48,;2.17,-2.02,;3.32,-3.05,;4.78,-2.57,;5.1,-1.07,;6.57,-.59,;7.71,-1.62,;6.89,.92,;3,-4.55,;1.53,-5.03,;.39,-4,;.71,-2.49,;-5.14,-1,;-4.74,-2.49,;-3.48,-2.83,;-5.83,-3.58,;-7.31,-3.18,;-7.71,-1.69,;-6.62,-.6,;-7.02,.88,;3.92,3.14,;5.01,2.06,;4.32,4.63,;5.81,5.03,)|