Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Neuraminidase
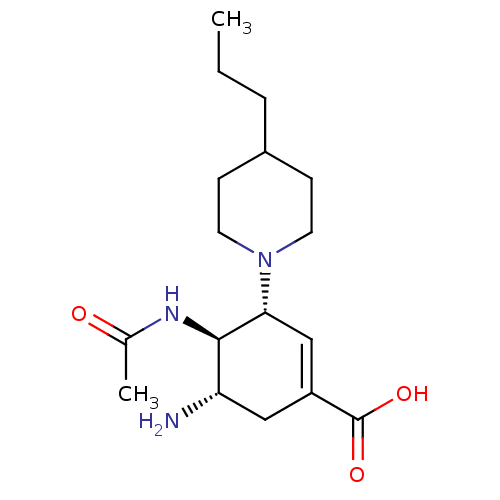
Ligand
BDBM5261
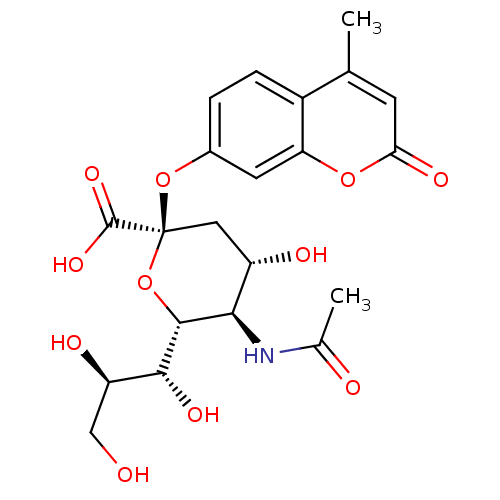
Substrate
BDBM4702
Meas. Tech.
Neuraminidase Inhibition Assay
IC50
87±n/a nM
Citation
 Lew, W; Wu, H; Chen, X; Graves, BJ; Escarpe, PA; MacArthur, HL; Mendel, DB; Kim, CU Carbocyclic influenza neuraminidase inhibitors possessing a C3-cyclic amine side chain: synthesis and inhibitory activity. Bioorg Med Chem Lett 10:1257-60 (2000) [PubMed] Article
Lew, W; Wu, H; Chen, X; Graves, BJ; Escarpe, PA; MacArthur, HL; Mendel, DB; Kim, CU Carbocyclic influenza neuraminidase inhibitors possessing a C3-cyclic amine side chain: synthesis and inhibitory activity. Bioorg Med Chem Lett 10:1257-60 (2000) [PubMed] Article More Info.:
Target
Name:
Neuraminidase
Synonyms:
Influenza B Virus Neuraminidase | NA | NRAM_INBLE | Neuraminidase | Neuraminidase B
Type:
Enzyme
Mol. Mass.:
51446.67
Organism:
Influenza B virus (B/Lee/40)
Description:
n/a
Residue:
466
Sequence:
MLPSTVQTLTLLLTSGGVLLSLYVSASLSYLLYSDVLLKFSSTKTTAPTMSLECTNASNAQTVNHSATKEMTFPPPEPEWTYPRLSCQGSTFQKALLISPHRFGEIKGNSAPLIIREPFVACGPKECRHFALTHYAAQPGGYYNGTRKDRNKLRHLVSVKLGKIPTVENSIFHMAAWSGSACHDGREWTYIGVDGPDNDALVKIKYGEAYTDTYHSYAHNILRTQESACNCIGGDCYLMITDGSASGISKCRFLKIREGRIIKEILPTGRVEHTEECTCGFASNKTIECACRDNSYTAKRPFVKLNVETDTAEIRLMCTKTYLDTPRPDDGSIAGPCESNGDKWLGGIKGGFVHQRMASKIGRWYSRTMSKTNRMGMELYVKYDGDPWTDSDALTLSGVMVSIEEPGWYSFGFEIKDKKCDVPCIGIEMVHDGGKDTWHSAATAIYCLMGSGQLLWDTVTGVDMAL
Inhibitor
Name:
BDBM5261
Synonyms:
(3R,4R,5S)-5-amino-4-acetamido-3-(4-propylpiperidin-1-yl)cyclohex-1-ene-1-carboxylic acid | C3-Cyclic Amine Carbocyclic Analogue 4l
Type:
Small organic molecule
Emp. Form.:
C17H29N3O3
Mol. Mass.:
323.4305
SMILES:
CCCC1CCN(CC1)[C@@H]1C=C(C[C@H](N)[C@H]1NC(C)=O)C(O)=O |r,c:11|
Substrate
Name:
BDBM4702
Synonyms:
(2R,4S,5R,6R)-5-acetamido-4-hydroxy-2-[(4-methyl-2-oxo-2H-chromen-7-yl)oxy]-6-[(1R,2R)-1,2,3-trihydroxypropyl]oxane-2-carboxylic acid | 2 -(4-methylumbelliferyl)-alpha-D-acetylneuraminic acid | neuraminidase substrate
Type:
Small organic molecule
Emp. Form.:
C21H25NO11
Mol. Mass.:
467.4233
SMILES:
CC(=O)N[C@@H]1[C@@H](O)C[C@](Oc2ccc3c(C)cc(=O)oc3c2)(O[C@H]1[C@H](O)[C@H](O)CO)C(O)=O |r|