Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Ephrin type-A receptor 2 [596-900]
Ligand
BDBM50299218
Substrate
n/a
Meas. Tech.
HotSpot Kinase Activity Assay
IC50
1.0±0.0 nM
Citation
 Heinzlmeir, S; Kudlinzki, D; Sreeramulu, S; Klaeger, S; Gande, SL; Linhard, V; Wilhelm, M; Qiao, H; Helm, D; Ruprecht, B; Saxena, K; Médard, G; Schwalbe, H; Kuster, B Chemical Proteomics and Structural Biology Define EPHA2 Inhibition by Clinical Kinase Drugs. ACS Chem Biol 11:3400-3411 (2016) [PubMed] Article
Heinzlmeir, S; Kudlinzki, D; Sreeramulu, S; Klaeger, S; Gande, SL; Linhard, V; Wilhelm, M; Qiao, H; Helm, D; Ruprecht, B; Saxena, K; Médard, G; Schwalbe, H; Kuster, B Chemical Proteomics and Structural Biology Define EPHA2 Inhibition by Clinical Kinase Drugs. ACS Chem Biol 11:3400-3411 (2016) [PubMed] Article More Info.:
Target
Name:
Ephrin type-A receptor 2 [596-900]
Synonyms:
ECK | EPHA2 | EPHA2_HUMAN | Ephrin type-A receptor 2 (EPHA2)
Type:
Protein
Mol. Mass.:
34367.96
Organism:
Homo sapiens (Human)
Description:
EPHA2 truncation (596-900 aa); 519U
Residue:
305
Sequence:
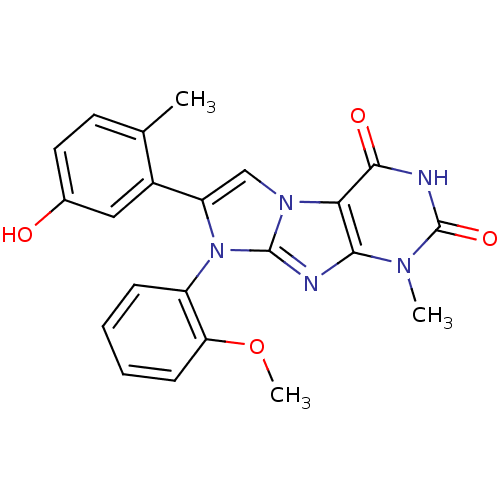
DPNQAVLKFTTEIHPSCVTRQKVIGAGEFGEVYKGMLKTSSGKKEVPVAIKTLKAGYTEKQRVDFLGEAGIMGQFSHHNIIRLEGVISKYKPMMIITEYMENGALDKFLREKDGEFSVLQLVGMLRGIAAGMKYLANMNYVHRDLAARNILVNSNLVCKVSDFGLSRVLEDDPEATYTTSGGKIPIRWTAPEAISYRKFTSASDVWSFGIVMWEVMTYGERPYWELSNHEVMKAINDGFRLPTPMDCPSAIYQLMMQCWQQERARRPKFADIVSILDKLIRAPDSLKTLADFDPRVSIRLPSTSG
Inhibitor
Name:
BDBM50299218
Synonyms:
8-(2-Methoxyphenyl)-1-methyl-7-(2'-methyl-5'-hydroxyphenyl-1H-imidazo[2,1-f]purine-2,4(3H,8H)-dione | CHEMBL566515 | Cpd66
Type:
Small organic molecule
Emp. Form.:
C22H19N5O4
Mol. Mass.:
417.4174
SMILES:
COc1ccccc1-n1c(cn2c1nc1n(C)c(=O)[nH]c(=O)c21)-c1cc(O)ccc1C |(16.89,-42.99,;16.89,-41.45,;18.23,-40.68,;19.55,-41.45,;20.88,-40.68,;20.88,-39.14,;19.55,-38.38,;18.23,-39.15,;16.9,-38.38,;16.91,-36.84,;15.44,-36.35,;14.53,-37.6,;15.43,-38.85,;14.53,-40.09,;13.06,-39.61,;11.74,-40.37,;11.73,-41.91,;10.41,-39.61,;9.07,-40.38,;10.41,-38.07,;11.74,-37.29,;11.74,-35.75,;13.06,-38.07,;18.24,-36.06,;19.58,-36.83,;20.9,-36.05,;22.24,-36.81,;20.9,-34.51,;19.55,-33.75,;18.23,-34.53,;16.89,-33.77,)|