Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
UDP-glucuronosyltransferase 1A4
Ligand
BDBM31768
Substrate
Midazolam
Meas. Tech.
UDP-glucuronosyltransferase Activity Assay
IC50
5.8e+4± 4e+3 nM
Citation
More Info.:
Target
Name:
UDP-glucuronosyltransferase 1A4
Synonyms:
Bilirubin-specific UDPGT isozyme 2 | GNT1 | GNT1 | UD14_HUMAN | UDP-glucuronosyltransferase 1-4 | UDP-glucuronosyltransferase 1-D | UDP-glucuronosyltransferase 1A4 | UDPGT 1-4 | UGT-1D | UGT1 | UGT1*4 | UGT1-04 | UGT1.4 | UGT1A4 | UGT1D | Uridine-5'-diphosphoglucuronosyltransferase 1A4 | hUG-BR2
Type:
Enzyme
Mol. Mass.:
60042.50
Organism:
Homo sapiens (Human)
Description:
P22310
Residue:
534
Sequence:
MARGLQVPLPRLATGLLLLLSVQPWAESGKVLVVPTDGSPWLSMREALRELHARGHQAVVLTPEVNMHIKEEKFFTLTAYAVPWTQKEFDRVTLGYTQGFFETEHLLKRYSRSMAIMNNVSLALHRCCVELLHNEALIRHLNATSFDVVLTDPVNLCGAVLAKYLSIPAVFFWRYIPCDLDFKGTQCPNPSSYIPKLLTTNSDHMTFLQRVKNMLYPLALSYICHTFSAPYASLASELFQREVSVVDLVSYASVWLFRGDFVMDYPRPIMPNMVFIGGINCANGKPLSQEFEAYINASGEHGIVVFSLGSMVSEIPEKKAMAIADALGKIPQTVLWRYTGTRPSNLANNTILVKWLPQNDLLGHPMTRAFITHAGSHGVYESICNGVPMVMMPLFGDQMDNAKRMETKGAGVTLNVLEMTSEDLENALKAVINDKSYKENIMRLSSLHKDRPVEPLDLAVFWVEFVMRHKGAPHLRPAAHDLTWYQYHSLDVIGFLLAVVLTVAFITFKCCAYGYRKCLGKKGRVKKAHKSKTH
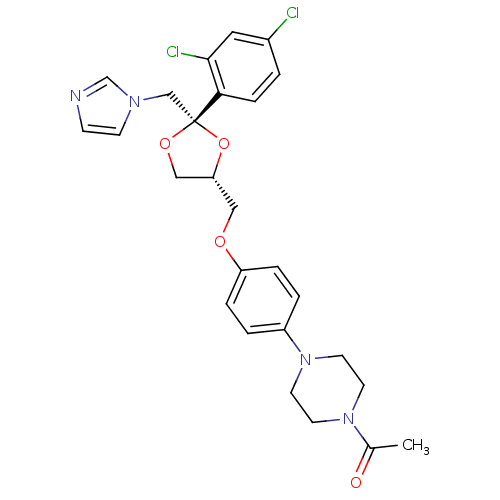
Inhibitor
Name:
BDBM31768
Synonyms:
CHEMBL295698 | Ketoconazole | Nizoral | Panfungol
Type:
Small organic molecule
Emp. Form.:
C26H28Cl2N4O4
Mol. Mass.:
531.431
SMILES:
CC(=O)N1CCN(CC1)c1ccc(OC[C@@H]2CO[C@](Cn3ccnc3)(O2)c2ccc(Cl)cc2Cl)cc1 |r|
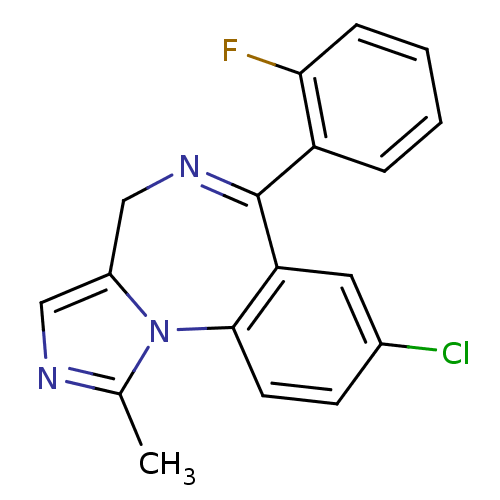
Substrate
Name:
BDBM21363
Synonyms:
12-chloro-9-(2-fluorophenyl)-3-methyl-2,4,8-triazatricyclo[8.4.0.0^{2,6}]tetradeca-1(10),3,5,8,11,13-hexaene | 8-chloro-6-(2-fluorophenyl)-1-methyl-4H-imidazo[1,5-a][1,4]benzodiazepine | CHEMBL655 | Dormicum | Midazolam | Ro 21-3981 | US20230416258, Compound Midazolam
Type:
Small organic molecule
Emp. Form.:
C18H13ClFN3
Mol. Mass.:
325.767
SMILES:
Cc1ncc2CN=C(c3ccccc3F)c3cc(Cl)ccc3-n12 |t:6|